Quick Navigation:
| | | |
Carbon and its Compounds
1. Introduction to Carbon
- Versatility: Carbon is a versatile element found in food, clothes, medicines, books, and all living structures.
- Occurrence:
- Earth's Crust: Contains only 0.02% carbon in the form of minerals (carbonates, hydrogen-carbonates, coal, petroleum).
- Atmosphere: Contains 0.03% carbon dioxide.
- Despite its small percentage in nature, its importance is immense.
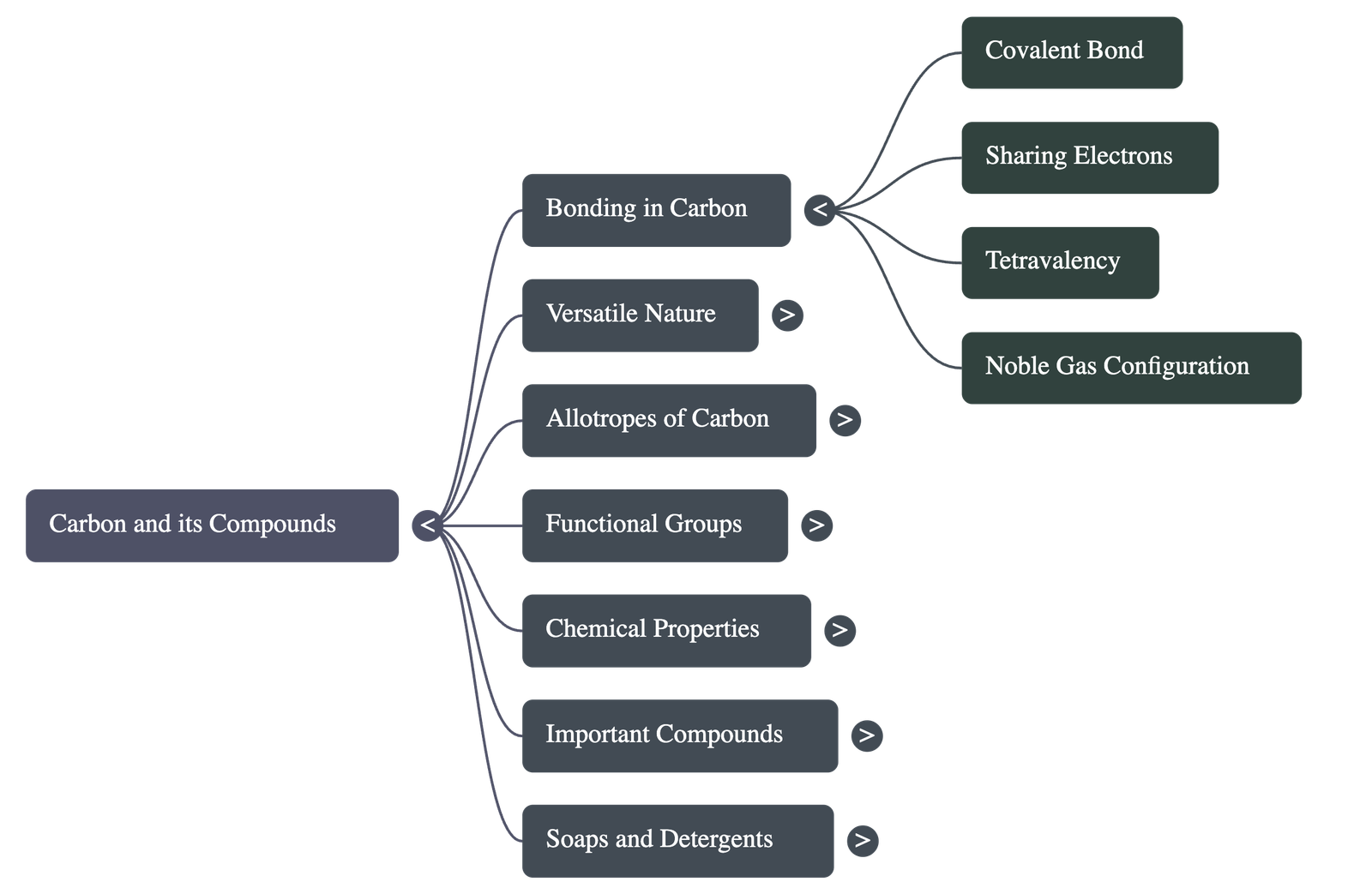
2. Bonding in Carbon – The Covalent Bond
Carbon forms covalent bonds rather than ionic bonds due to its atomic structure (Atomic Number: 6; Configuration: 2, 4).
- Why not Ionic?
- Gaining 4 electrons (C4-): It is difficult for the nucleus with 6 protons to hold on to 10 electrons.
- Losing 4 electrons (C4+): It requires a large amount of energy to remove 4 electrons.
- Solution (Sharing): Carbon shares valence electrons with other atoms to attain noble gas configuration. This sharing forms a Covalent Bond.
- Properties of Covalent Compounds:
- Low melting and boiling points (weak intermolecular forces).
- Poor conductors of electricity (no ions formed).
- Examples of Covalent Molecules:
- Hydrogen (H2): Single bond.
- Oxygen (O2): Double bond.
- Nitrogen (N2): Triple bond.
- Methane (CH4): Carbon shares 4 electrons with 4 Hydrogen atoms.
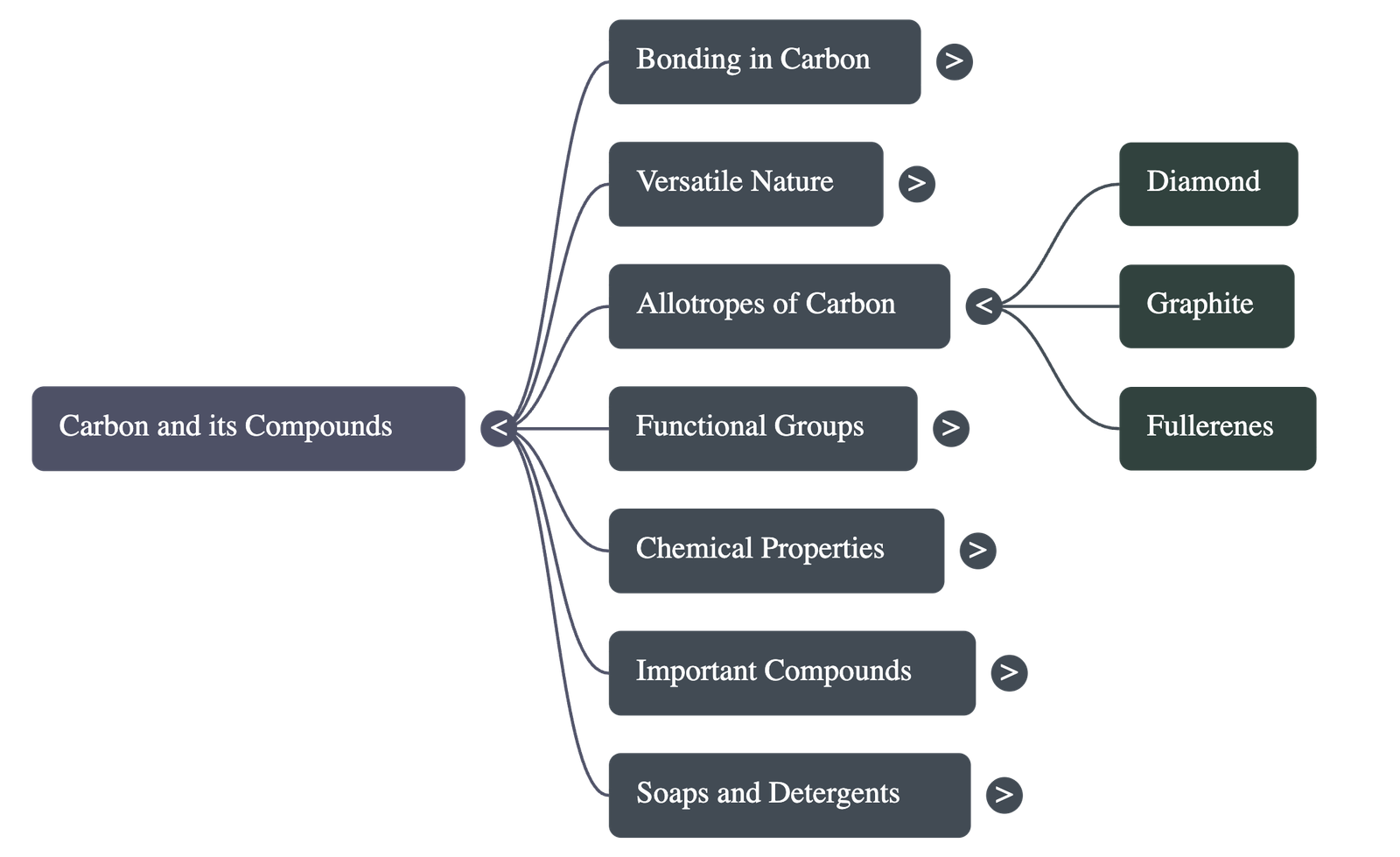
3. Allotropes of Carbon
The element carbon occurs in different physical forms known as allotropes:
- Diamond: Each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure. It is the hardest known substance and a poor conductor.
- Graphite: Each carbon atom is bonded to three others in the same plane, forming hexagonal arrays placed in layers. It is smooth, slippery, and a good conductor of electricity.
- Fullerenes: Carbon atoms arranged in the shape of a football (e.g., C-60).
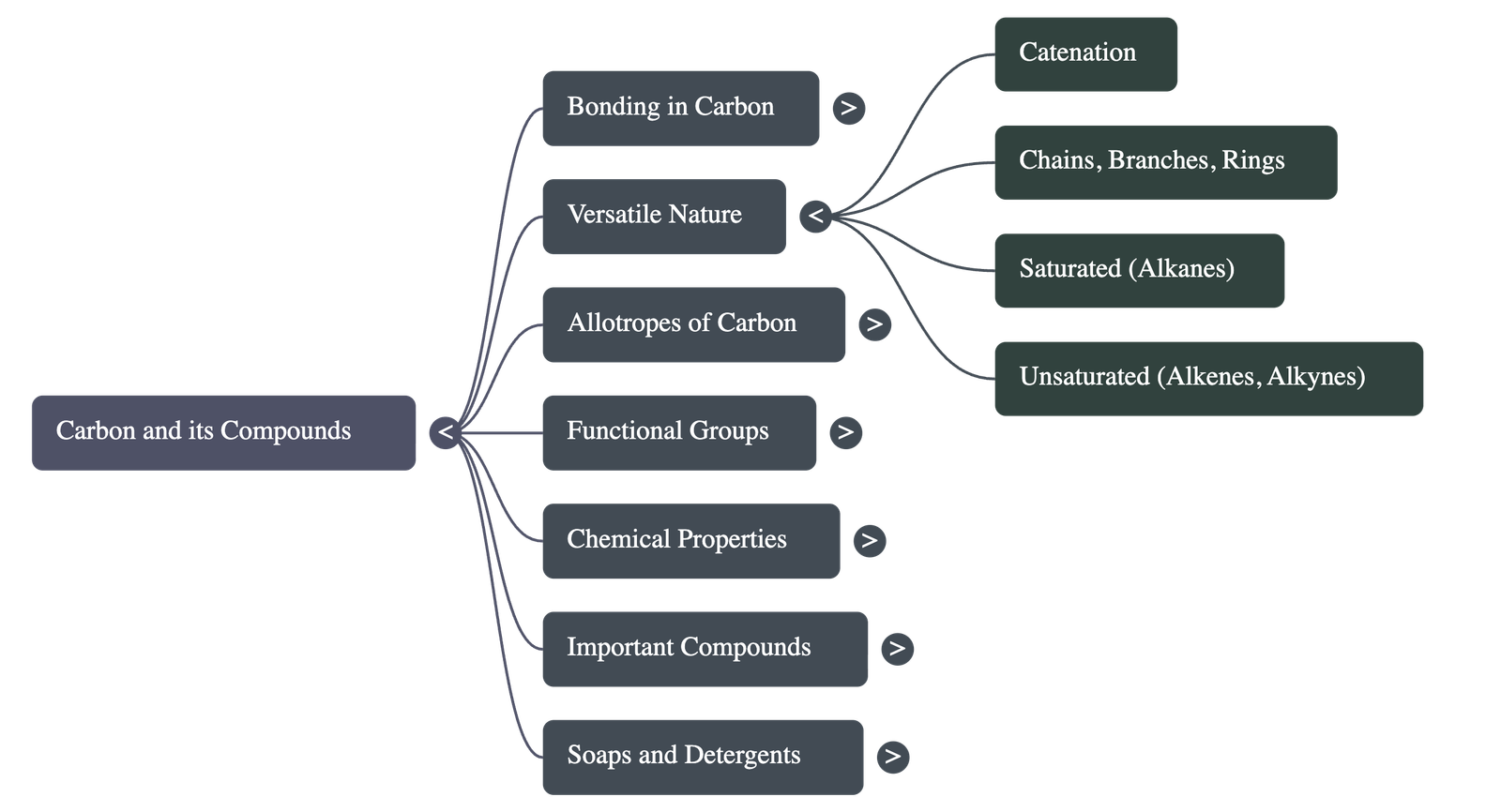
4. Versatile Nature of Carbon
Two unique factors allow carbon to form millions of compounds:
- Catenation: The unique ability of carbon to form bonds with other carbon atoms, giving rise to large molecules (long chains, branched chains, or rings). Carbon-Carbon bonds are very strong and stable.
- Tetravalency: With a valency of 4, carbon is capable of bonding with four other atoms of carbon or atoms of other mono-valent elements (like Oxygen, Hydrogen, Nitrogen, Sulphur, Chlorine).
5. Types of Carbon Compounds
Saturated vs. Unsaturated
- Saturated Compounds (Alkanes): Carbon atoms linked by single bonds only. They are generally not very reactive.
- Unsaturated Compounds (Alkenes/Alkynes): Carbon atoms linked by double or triple bonds. They are more reactive than saturated compounds.
Structural Forms
- Chains: Straight chains of carbon (e.g., Propane, Butane).
- Branches: Carbon skeletons that branch off.
- Isomers: Compounds with the identical molecular formula but different structures (e.g., n-butane and iso-butane).
- Rings: Carbon atoms arranged in a ring (e.g., Cyclohexane - saturated; Benzene - unsaturated).
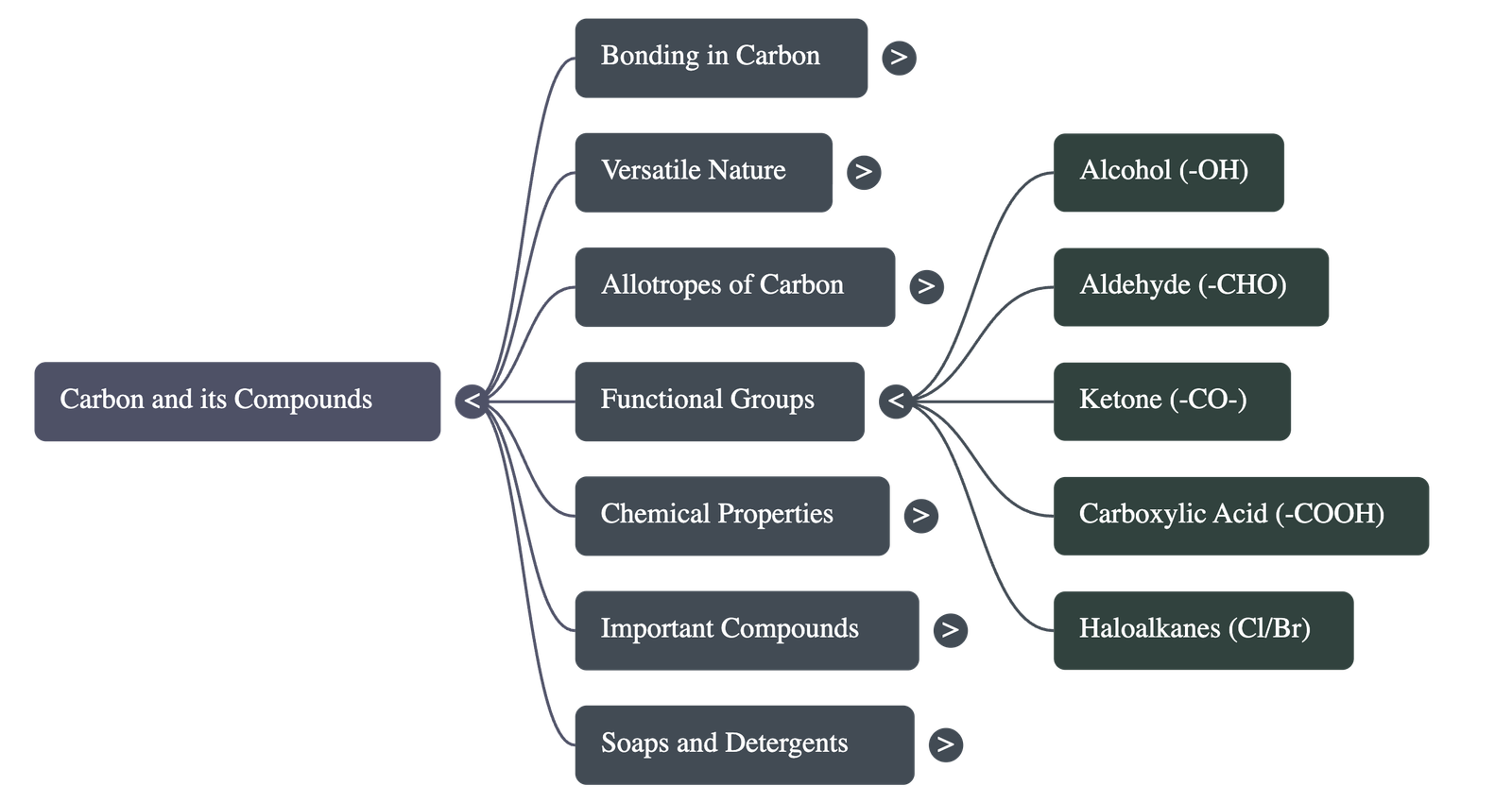
6. Functional Groups and Homologous Series
- Heteroatoms: Elements like oxygen, nitrogen, or halogens that replace hydrogen in a hydrocarbon chain.
- Functional Groups: Specific groups of atoms that confer specific properties to the compound, regardless of carbon chain length.
- Halogens: Chloro-, Bromo-
- Alcohol: -OH
- Aldehyde: -CHO
- Ketone: -CO-
- Carboxylic Acid: -COOH
- Homologous Series: A series of compounds in which the same functional group substitutes for hydrogen in a carbon chain.
- Successive members differ by a –CH2– unit.
- Successive members differ by 14 u in molecular mass.
- Properties: Chemical properties remain similar; physical properties (like melting/boiling points) show a gradation (increase with mass).
7. Chemical Properties of Carbon Compounds
- Combustion:
- Burning in oxygen gives CO2, heat, and light.
- Saturated Hydrocarbons: Clean blue flame.
- Unsaturated Hydrocarbons: Yellow sooty flame (black smoke).
- Note: Even saturated hydrocarbons give a sooty flame if air supply is limited.
- Oxidation:
- Alcohols can be converted to carboxylic acids using oxidising agents like alkaline KMnO4 or acidified K2Cr2O7.
- Addition Reaction:
- Unsaturated hydrocarbons add hydrogen in the presence of catalysts (Pd or Ni) to become saturated.
- Used to hydrogenate vegetable oils (liquid) into vegetable ghee (solid).
- Substitution Reaction:
- In sunlight, chlorine replaces hydrogen atoms in saturated hydrocarbons one by one.
8. Important Carbon Compounds
Ethanol (Alcohol)
- Liquid at room temperature, good solvent, soluble in water.
- Reaction with Sodium: Produces Sodium Ethoxide and Hydrogen gas.
- Dehydration: Heating with concentrated H2SO4 removes water to form Ethene.
Ethanoic Acid (Acetic Acid)
- 5-8% solution in water is Vinegar.
- Pure acid freezes in winter (Glacial Acetic Acid). It is a weak acid.
- Esterification: Reacts with alcohol to form sweet-smelling Esters (used in perfumes/flavoring).
- Saponification: Esters react with alkali to give back alcohol and sodium salt of acid (soap).
- Reaction with Carbonates/Bicarbonates: Produces salt, water, and Carbon Dioxide (brisk effervescence).
9. Soaps and Detergents
- Soap Molecules: Sodium or potassium salts of long-chain carboxylic acids.
- Ionic End: Hydrophilic (dissolves in water).
- Carbon Chain: Hydrophobic (dissolves in oil).
- Cleaning Action (Micelles):
- In water, soap forms structures called micelles.
- The hydrophobic tail attaches to dirt (oil), and the ionic head faces outward into the water.
- This forms an emulsion, allowing dirt to be washed away.
- Scum: In hard water (containing Ca and Mg salts), soap forms an insoluble precipitate called scum, making it ineffective.
- Detergents: Ammonium or sulphonate salts of long-chain carboxylic acids. They do not form insoluble precipitates with Ca and Mg ions, making them effective in hard water.
Quick Navigation:
| | | |
1 / 1
Quick Navigation:
| | | |