Quick Navigation:
| | | |
Acids, Bases and Salts
Introduction
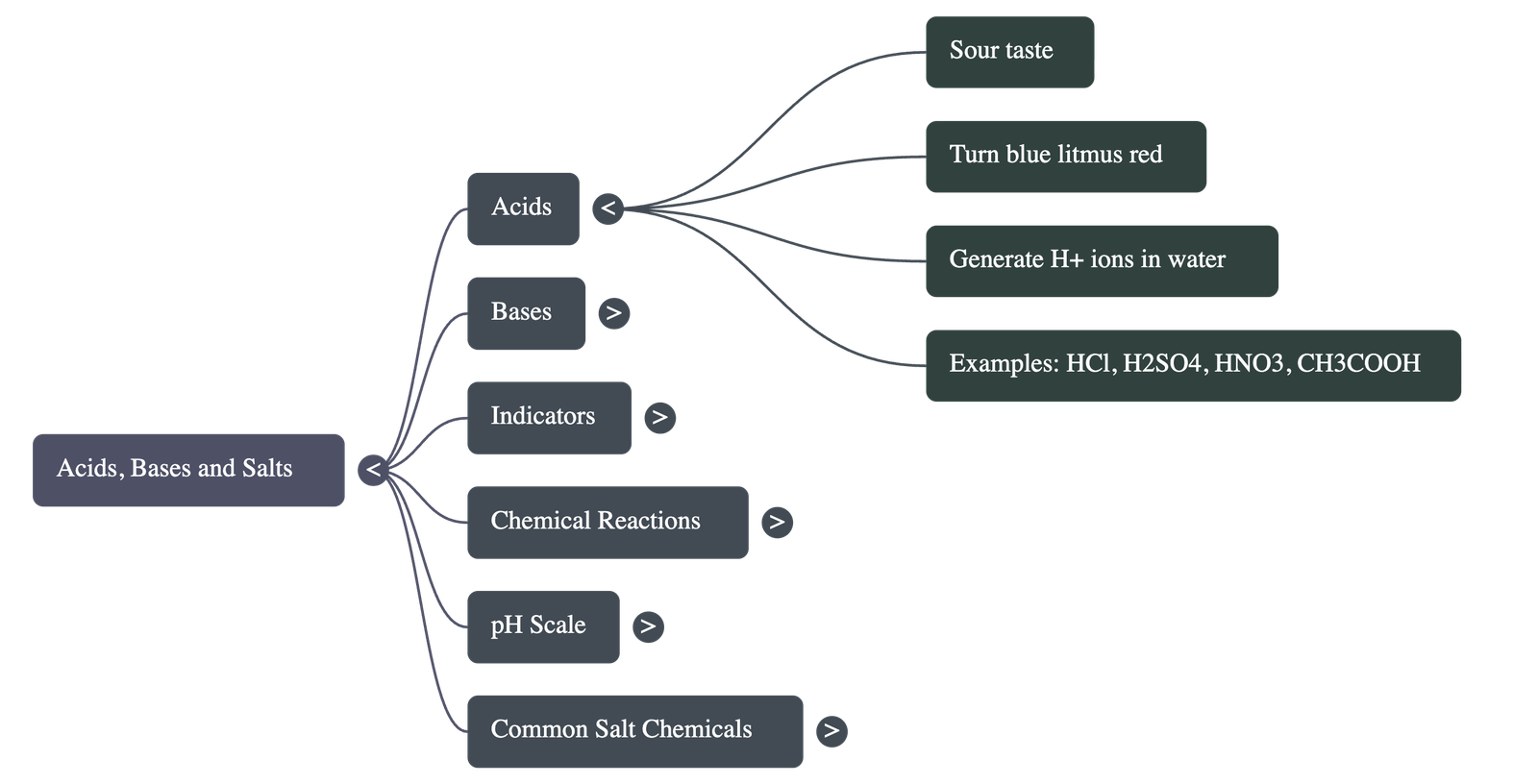
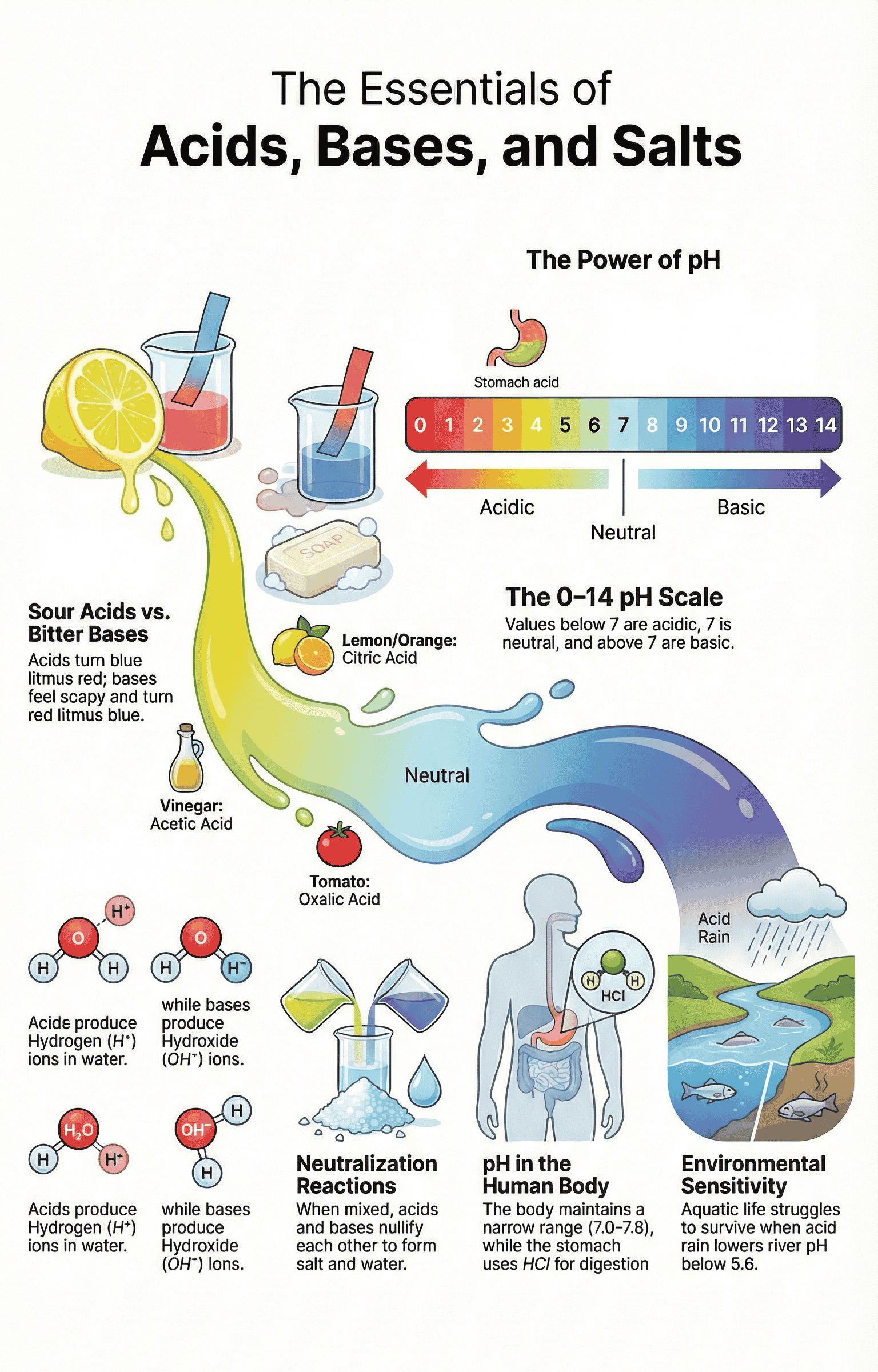
- Acids: Substances that taste sour and turn blue litmus paper red.
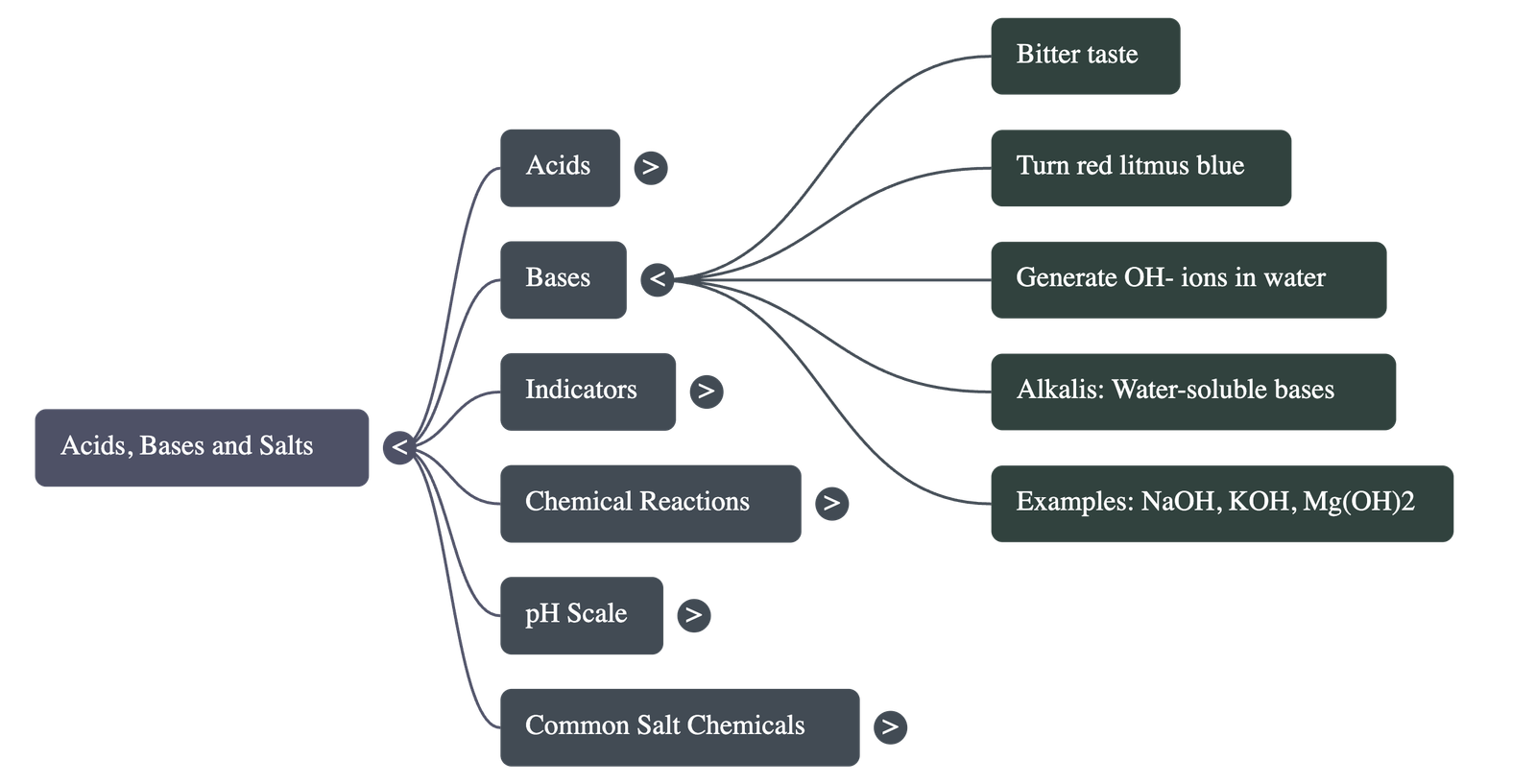
- Bases: Substances that taste bitter and turn red litmus paper blue.
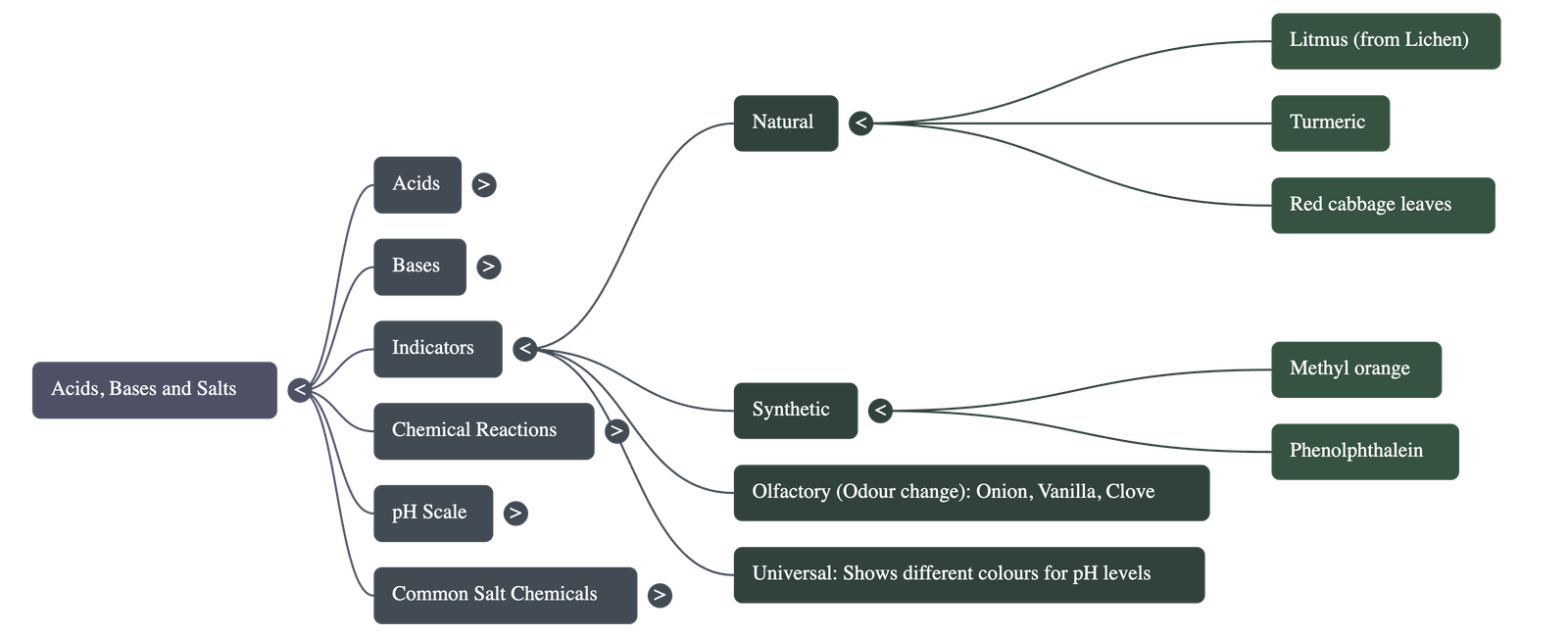
- Indicators: Substances used to test whether a solution is acidic or basic.
- Natural Indicators: Litmus (extracted from lichen), turmeric (turns reddish-brown in base), red cabbage leaves, and certain flower petals like Hydrangea, Petunia, and Geranium.
- Synthetic Indicators: Methyl orange and phenolphthalein.
- Olfactory Indicators: Substances whose odour changes in acidic or basic media (e.g., vanilla, onion, clove).
Chemical Properties of Acids and Bases
Reaction with Metals
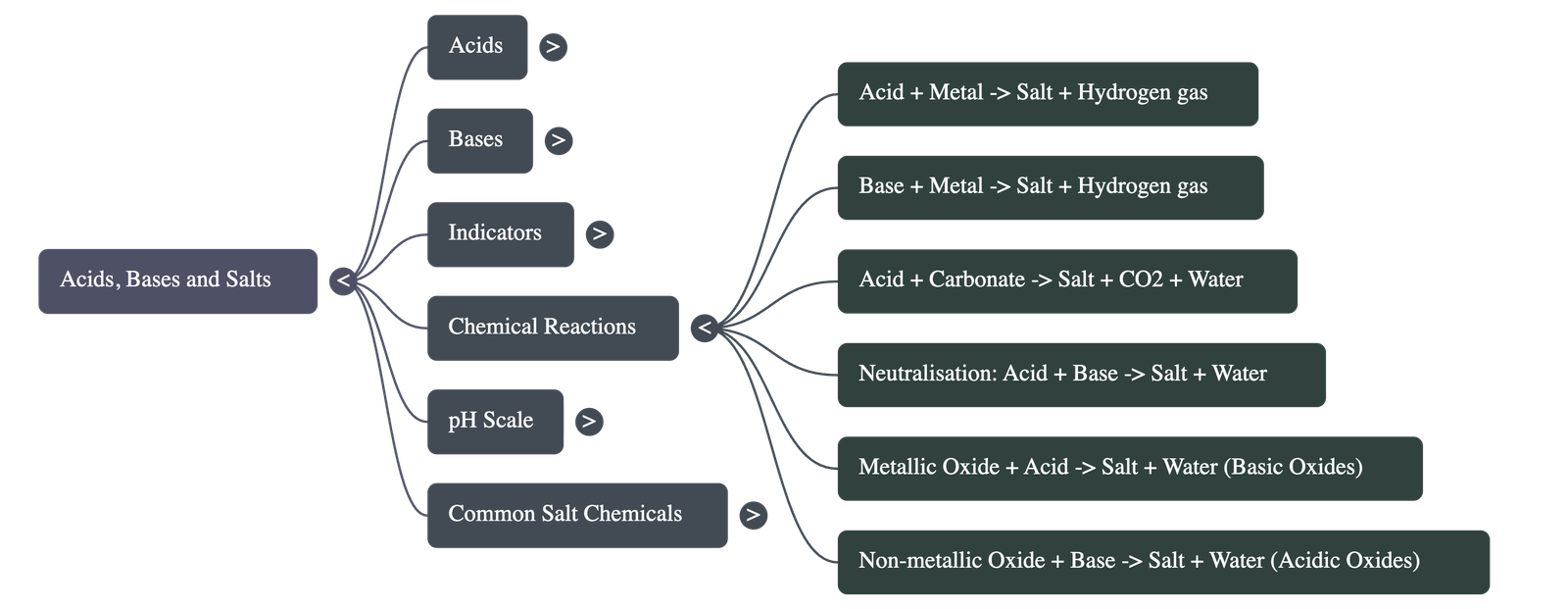
- Acid + Metal → Salt + Hydrogen gas
- Metals displace hydrogen from acids. The evolved hydrogen gas burns with a "pop" sound.
- Base + Metal: Alkalis like sodium hydroxide also react with metals like zinc to produce hydrogen gas and a salt (e.g., Sodium Zincate). Note: Not all metals react with bases.
Reaction with Metal Carbonates and Hydrogencarbonates
- Metal Carbonate/Hydrogencarbonate + Acid → Salt + Carbon Dioxide + Water
- Carbon dioxide gas turns lime water milky due to the formation of calcium carbonate (white precipitate).
- If excess carbon dioxide is passed through the milky lime water, it becomes clear again due to the formation of soluble calcium hydrogencarbonate.
Neutralisation Reaction
- Acid + Base → Salt + Water
- Acids and bases nullify each other's effects.
Reactions of Oxides
- Metallic Oxides + Acid → Salt + Water: Metallic oxides are basic in nature (e.g., Copper oxide reacting with HCl to form blue-green Copper(II) chloride).
- Non-metallic Oxides + Base → Salt + Water: Non-metallic oxides are acidic in nature (e.g., Carbon dioxide reacting with Calcium hydroxide).
Common Characteristics of Acids and Bases
- Electrical Conductivity: Acidic and basic solutions conduct electricity because they produce ions in water.
- Acids in Water: Produce H⁺(aq) ions (Hydrogen ions). H⁺ ions cannot exist alone; they combine with water to form Hydronium ions (H₃O⁺).
- Bases in Water: Produce OH⁻(aq) ions (Hydroxide ions). Bases soluble in water are called Alkalis.
- Dilution: Mixing an acid or base with water decreases the concentration of ions (H₃O⁺ or OH⁻) per unit volume.
- Exothermic Process: Dissolving acid or base in water releases a lot of heat.
- Safety Rule: Always add acid to water slowly with stirring. Never add water to concentrated acid to avoid splashing and breaking the container.
Strength of Acid and Base Solutions
- pH Scale: A scale (0–14) for measuring hydrogen ion concentration.
- pH = 7: Neutral solution.
- pH < 7: Acidic solution (Lower pH = stronger acid).
- pH > 7: Basic solution (Higher pH = stronger base).
- Universal Indicator: A mixture of several indicators that shows different colours at different pH values.
- Strong vs. Weak:
- Strong Acids/Bases: Ionize completely/produce large amounts of H⁺ or OH⁻ ions (e.g., HCl, NaOH).
- Weak Acids/Bases: Ionize partially/produce small amounts of H⁺ or OH⁻ ions (e.g., Acetic acid, Ammonium hydroxide).
Importance of pH in Everyday Life
- Biological Systems: Our body works within a pH range of 7.0 to 7.8. Aquatic life suffers if rain water pH drops below 5.6 (Acid Rain).
- Soil: Plants require a specific pH range for healthy growth.
- Digestion: The stomach produces Hydrochloric acid for digestion. Excess acid causes indigestion, which is treated with Antacids (mild bases like Magnesium hydroxide/Milk of Magnesia).
- Tooth Decay: Starts when mouth pH is lower than 5.5. Bacteria produce acid by degrading sugar. Toothpaste (basic) neutralizes this acid.
- Self-Defence in Nature:
- Bee sting: Leaves an acid causing pain; treated with mild base like baking soda.
- Nettle leaves: Inject methanoic acid causing burning pain; treated with dock plant leaves.
More About Salts
Family and pH of Salts
- Salts of Strong Acid + Strong Base = Neutral (pH 7).
- Salts of Strong Acid + Weak Base = Acidic (pH < 7).
- Salts of Weak Acid + Strong Base = Basic (pH > 7).
Chemicals from Common Salt (NaCl)
Common salt is a raw material for various chemicals:
- Sodium Hydroxide (NaOH):
Produced by the Chlor-alkali process (Electrolysis of brine/salt water).
Products: Chlorine (Anode), Hydrogen (Cathode), NaOH (near Cathode). - Bleaching Powder (CaOCl₂):
Produced by the action of chlorine gas on dry slaked lime [Ca(OH)₂].
Uses: Bleaching cotton/linen/wood pulp, oxidising agent, disinfecting drinking water. - Baking Soda (NaHCO₃):
Chemical name: Sodium hydrogencarbonate.
Produced using NaCl, H₂O, CO₂ and NH₃.
Properties: Mild non-corrosive base. Releases CO₂ upon heating (makes cakes spongy).
Uses: Antacids, soda-acid fire extinguishers, making baking powder (mixture of baking soda and mild edible acid like tartaric acid). - Washing Soda (Na₂CO₃·10H₂O):
Obtained by recrystallisation of sodium carbonate.
Uses: Glass, soap, and paper industries; cleaning agent; removing permanent hardness of water.
Are Salt Crystals Dry?
- Water of Crystallisation: The fixed number of water molecules present in one formula unit of a salt (e.g., CuSO₄·5H₂O is blue; heating it removes water turning it white).
- Plaster of Paris (CaSO₄·½H₂O):
Calcium sulphate hemihydrate. Obtained by heating Gypsum (CaSO₄·2H₂O) at 373 K.
Uses: Plaster for fractured bones, making toys, decoration materials.
Reaction: Mixes with water to form hard solid Gypsum again.
Quick Navigation:
| | | |
1 / 1
Quick Navigation:
| | | |