Quick Navigation:
| | | |
Chemical Reactions and Equations
1. Introduction to Chemical Reactions
- Chemical Change: Whenever a chemical change occurs, a chemical reaction has taken place. The nature and identity of the initial substance change.
- Examples in Daily Life: Souring of milk, rusting of iron, fermentation of grapes, cooking of food, digestion, and respiration.
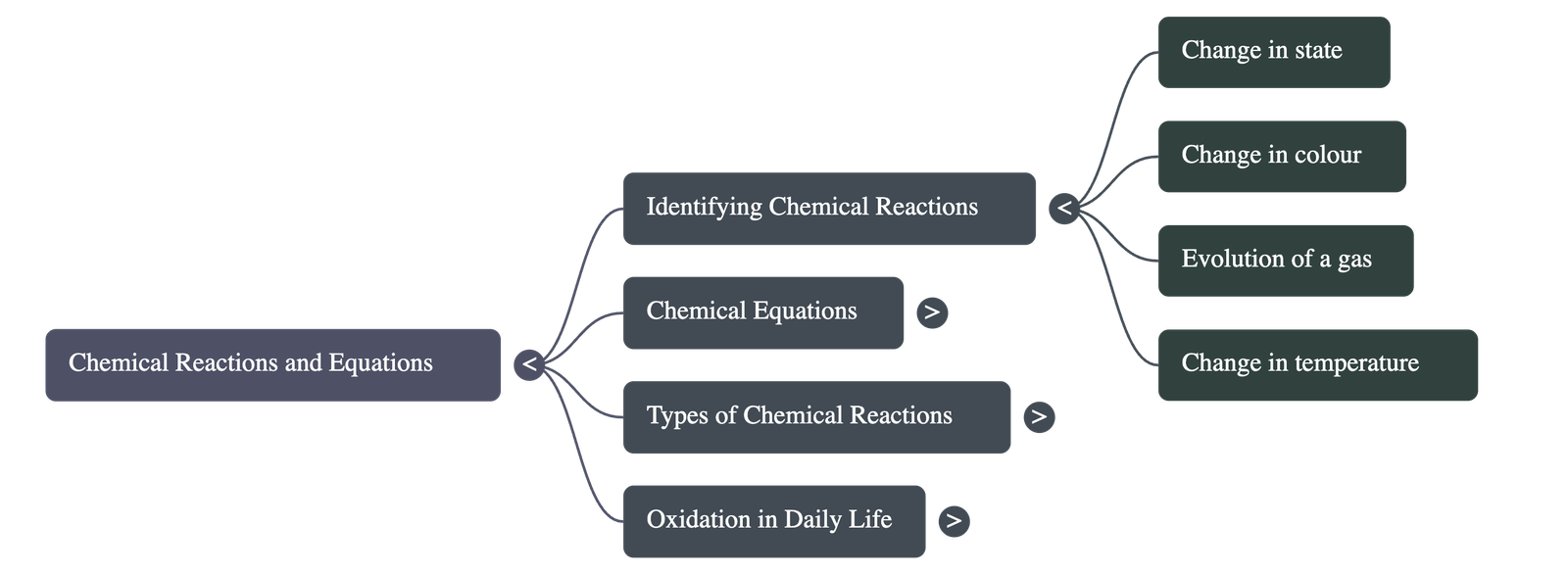
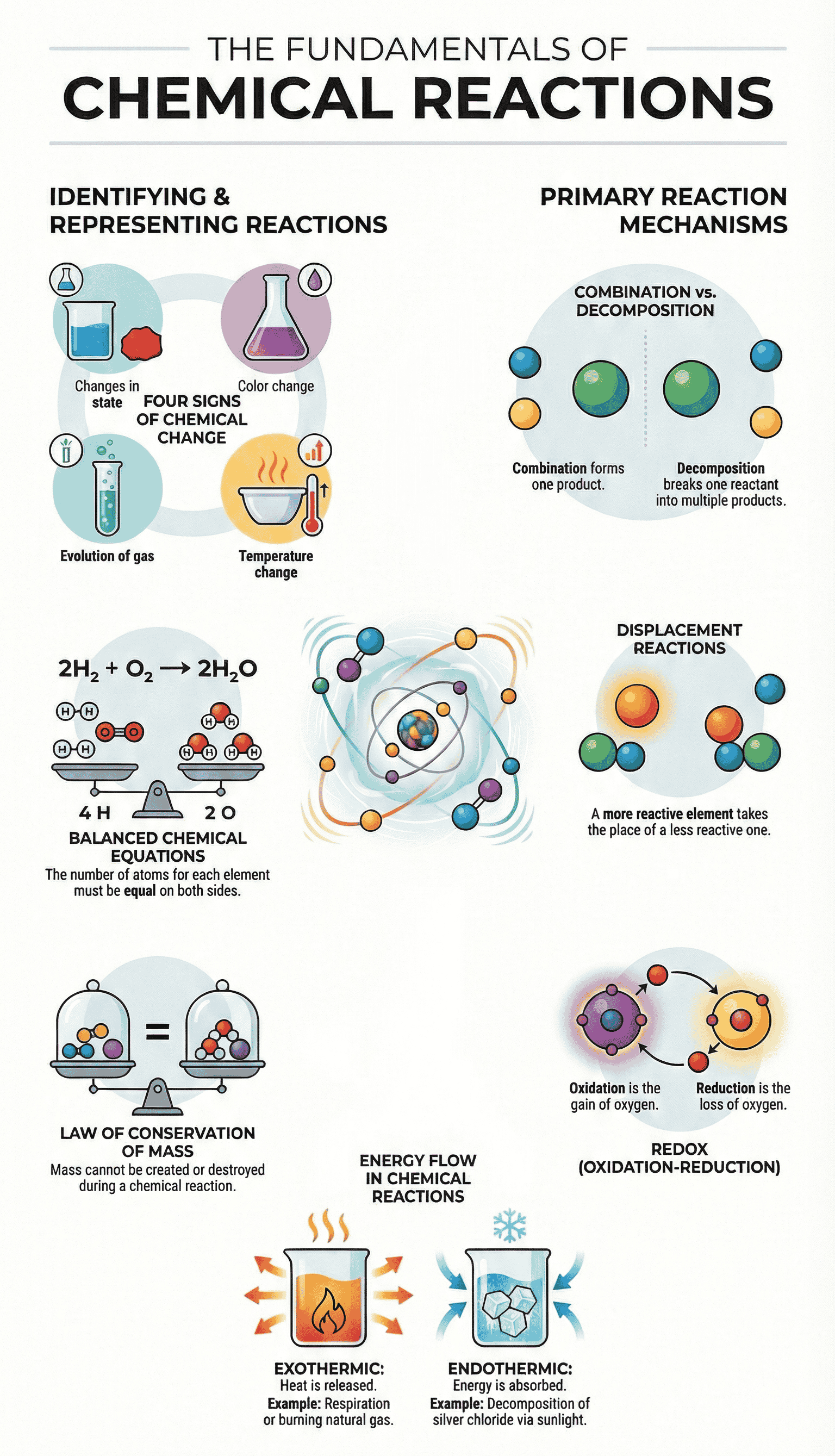
- Indicators of a Chemical Reaction: A reaction can be identified by:
- Change in state (solid, liquid, gas).
- Change in colour.
- Evolution of a gas.
- Change in temperature.
- Activity Example: Burning a magnesium ribbon in air produces a dazzling white flame and changes into a white powder called magnesium oxide.
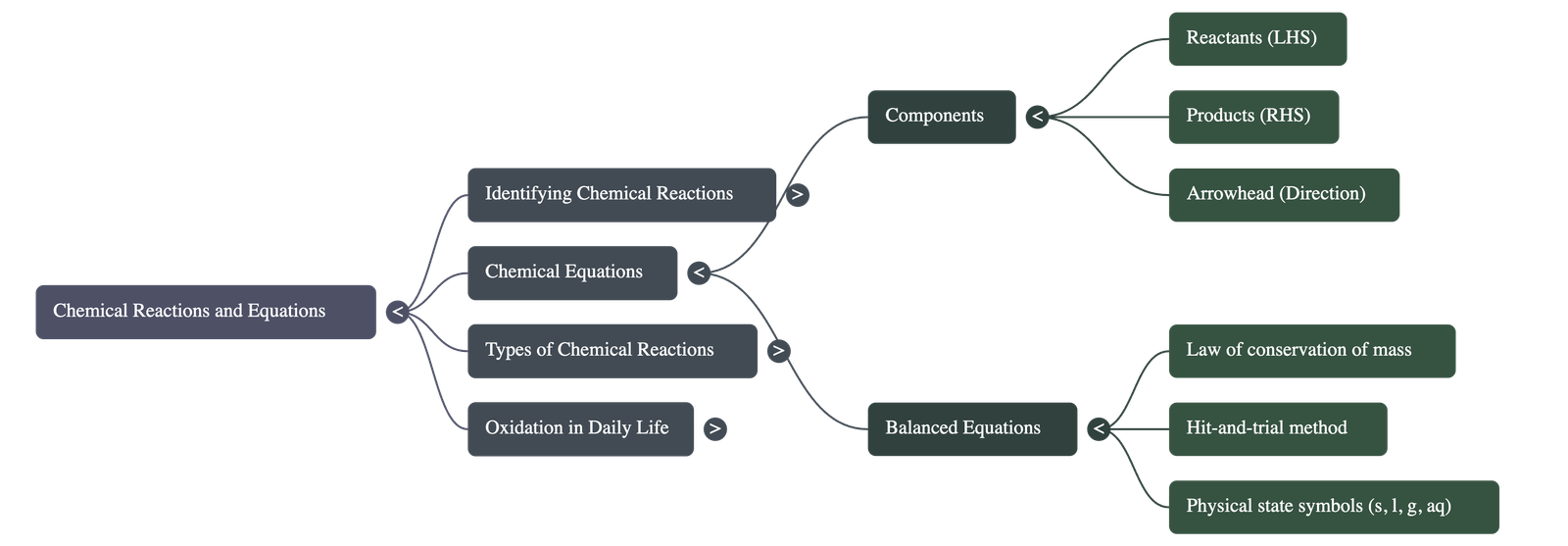
2. Chemical Equations
- Word Equations: The simplest way to describe a reaction.
Example: Magnesium + Oxygen → Magnesium oxide.
Reactants are on the left-hand side (LHS) and products are on the right-hand side (RHS). - Skeletal Chemical Equation: Uses chemical formulae instead of words. If the mass is not the same on both sides, it is an unbalanced or skeletal equation.
Example: Mg + O2 → MgO - Balanced Chemical Equations: Based on the Law of Conservation of Mass (mass can neither be created nor destroyed). The number of atoms of each element must remain the same before and after the reaction.
- Balancing Method: Equations are balanced using the "hit-and-trial" method by adjusting whole number coefficients.
- Physical States: To make equations informative, physical states are noted: (g) for gas, (l) for liquid, (s) for solid, and (aq) for aqueous (solution in water).
- Reaction Conditions: Temperature, pressure, and catalysts are often written above or below the arrow.
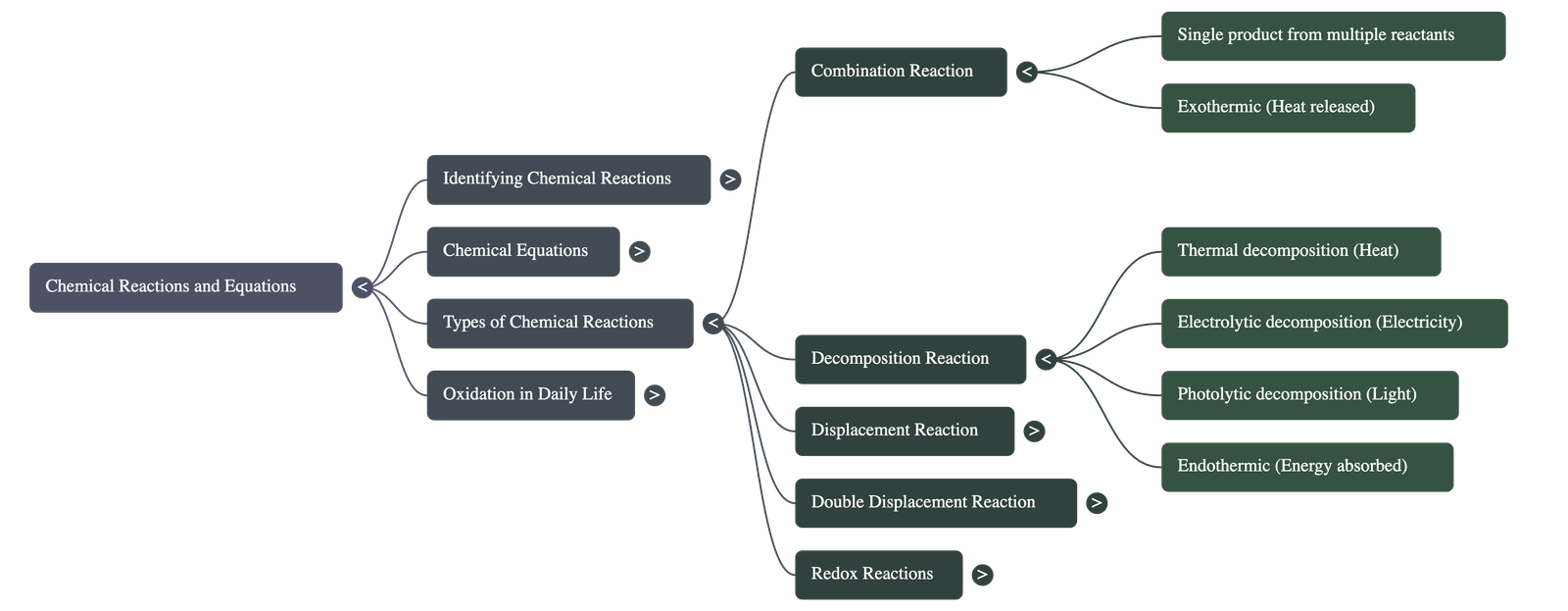
3. Types of Chemical Reactions
A. Combination Reaction
- Definition: A reaction in which two or more reactants combine to form a single product.
- Example: Calcium oxide (quick lime) reacts vigorously with water to form calcium hydroxide (slaked lime).
Equation: CaO(s) + H2O(l) → Ca(OH)2(aq) + Heat - Exothermic Reactions: Reactions that release heat along with products.
- Burning of natural gas.
- Respiration: Glucose combines with oxygen in cells to provide energy.
- Decomposition of vegetable matter into compost.
B. Decomposition Reaction
- Definition: A single reactant breaks down to give simpler products. These are generally Endothermic Reactions (require energy).
- Thermal Decomposition (using heat):
- Ferrous sulphate (green) loses water and decomposes to ferric oxide (brown), sulphur dioxide, and sulphur trioxide.
- Calcium carbonate (limestone) decomposes to calcium oxide (quick lime) and carbon dioxide (used in cement manufacture).
- Lead nitrate (white) decomposes to lead oxide, nitrogen dioxide (brown fumes), and oxygen.
- Electrolytic Decomposition (using electricity): Electrolysis of water produces hydrogen and oxygen gases.
- Photolytic Decomposition (using sunlight): Silver chloride or silver bromide turns grey in sunlight as they decompose into silver metal and chlorine/bromine gas. Used in black and white photography.
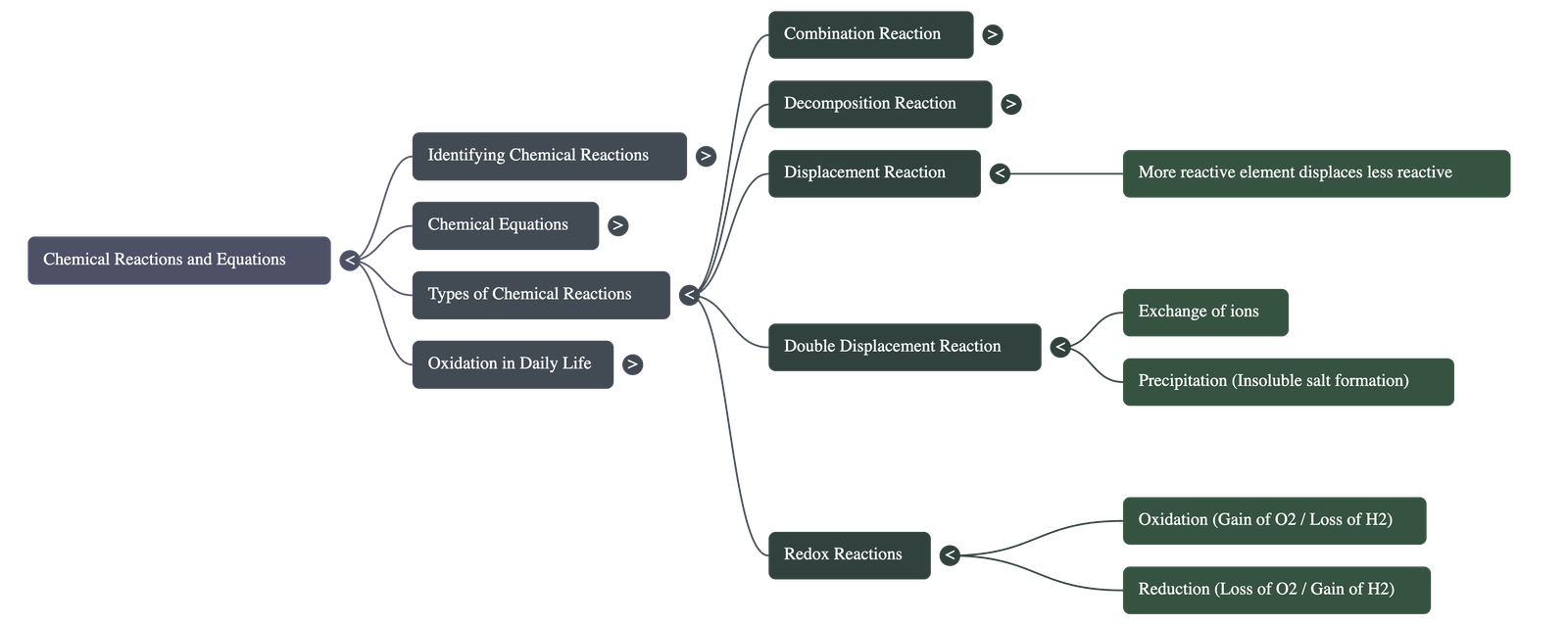
C. Displacement Reaction
- Definition: A more reactive element displaces or removes a less reactive element from its compound.
- Example: Iron nails dipped in copper sulphate solution turn brownish, and the blue solution fades. Iron displaces copper.
Equation: Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s) - Zinc and Lead are more reactive than Copper and also displace it.
D. Double Displacement Reaction
- Definition: Reactions in which there is an exchange of ions between the reactants.
- Precipitation Reaction: Any reaction that produces an insoluble substance (precipitate).
- Example: Sodium sulphate reacts with barium chloride to form a white precipitate of barium sulphate and sodium chloride.
Equation: Na2SO4 + BaCl2 → BaSO4 (precipitate) + 2NaCl
4. Oxidation and Reduction (Redox)
- Oxidation: The gain of oxygen or the loss of hydrogen by a substance.
- Reduction: The loss of oxygen or the gain of hydrogen by a substance.
- Redox Reaction: A reaction where one reactant gets oxidised while the other gets reduced simultaneously.
- Example: CuO + H2 → Cu + H2O
Explanation: Copper oxide loses oxygen (Reduced) and Hydrogen gains oxygen (Oxidised).
5. Effects of Oxidation in Everyday Life
Corrosion
- When a metal is attacked by substances like moisture or acids, it corrodes.
- Rusting of Iron: Iron acquires a coating of reddish-brown powder.
- Other examples include the black coating on silver and the green coating on copper.
- Corrosion causes significant damage to bridges, ships, and car bodies.
Rancidity
- When fats and oils are oxidised, they become rancid, leading to a change in smell and taste.
- Prevention:
- Adding antioxidants to foods containing fats and oils.
- Storing food in airtight containers to slow oxidation.
- Flushing bags of chips with gases like nitrogen to prevent oxidation.
Quick Navigation:
| | | |
1 / 1
Quick Navigation:
| | | |