Quick Navigation:
| | | |
Metals and Non-metals
1. Physical Properties
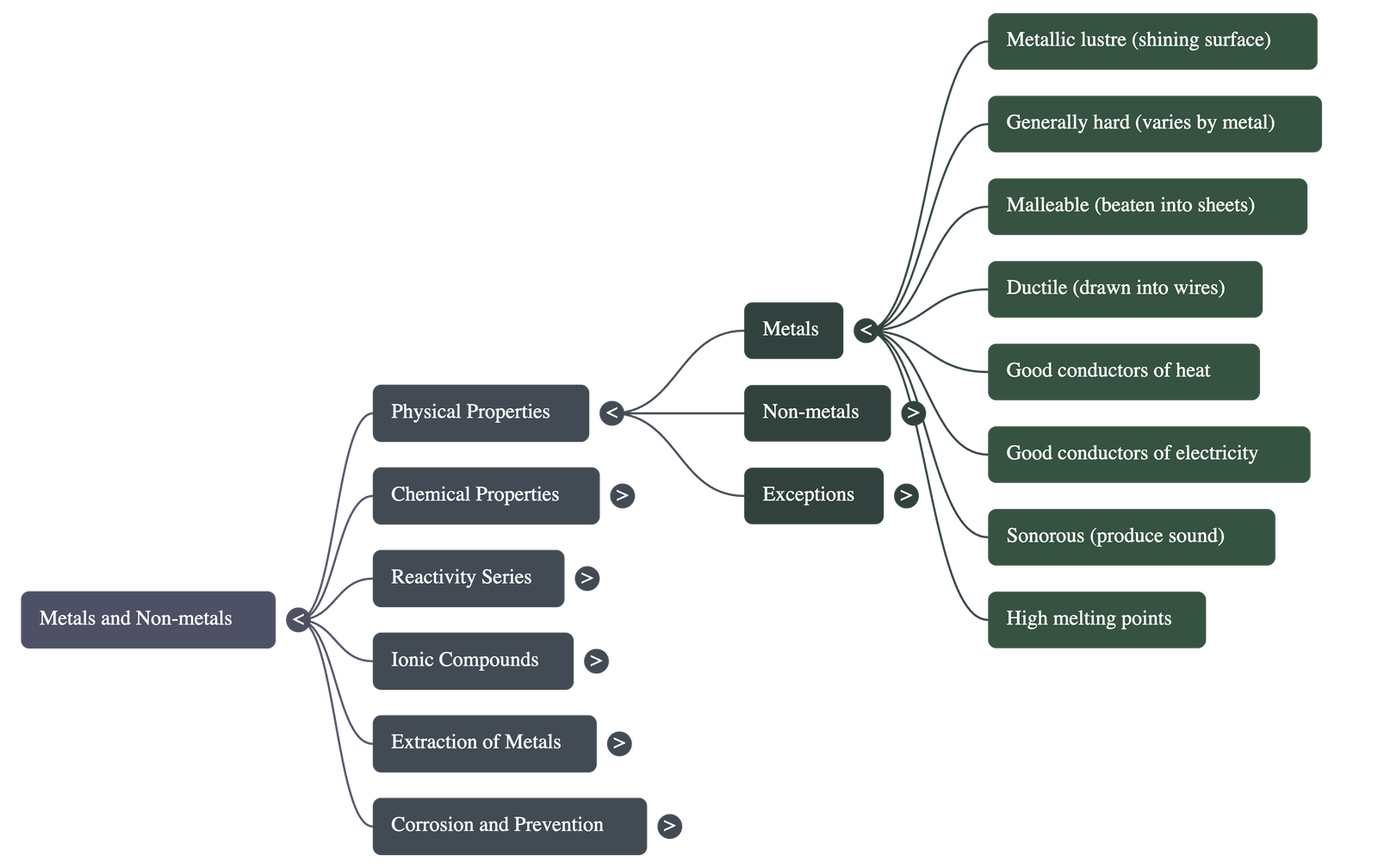
Metals
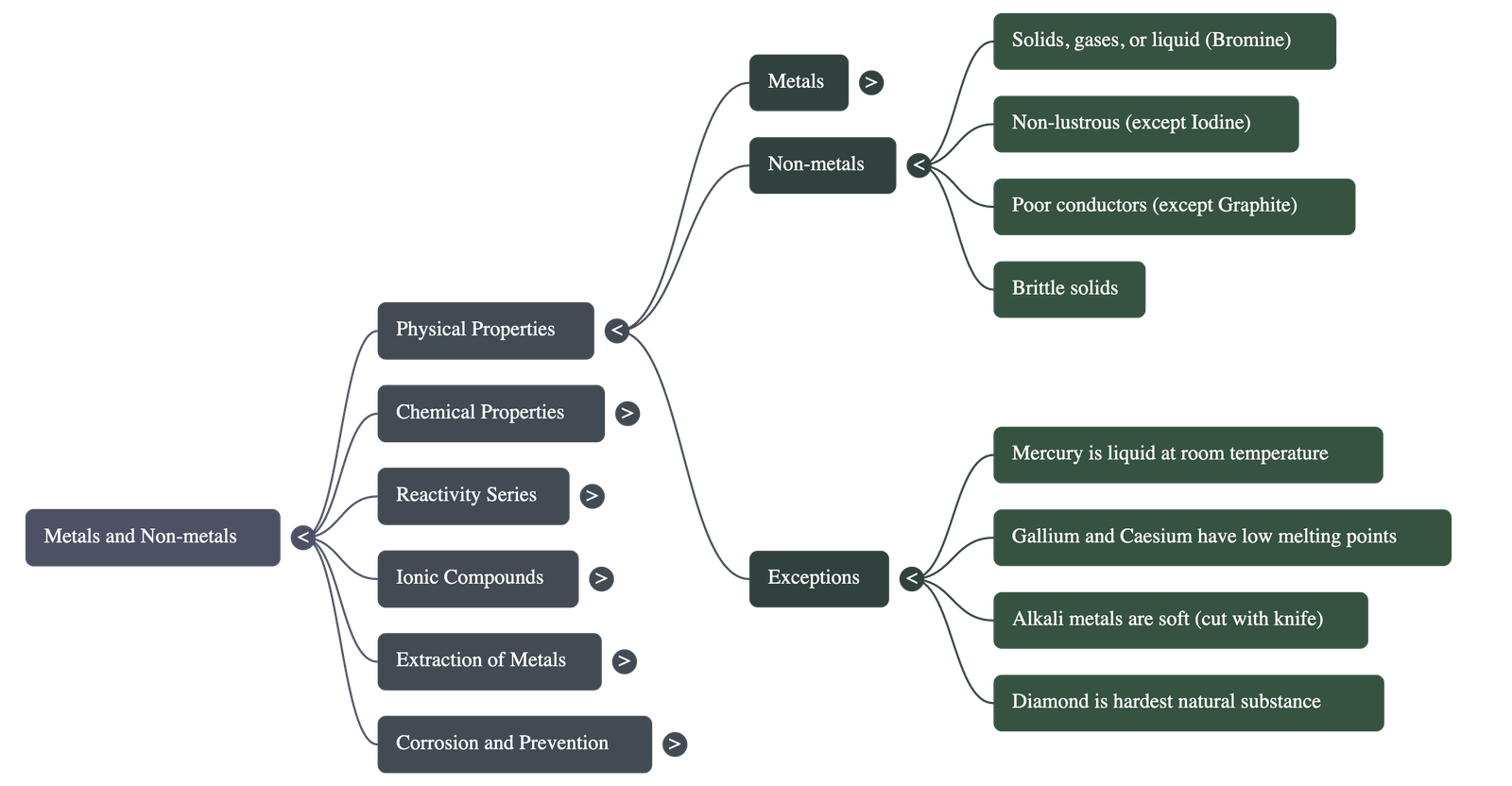
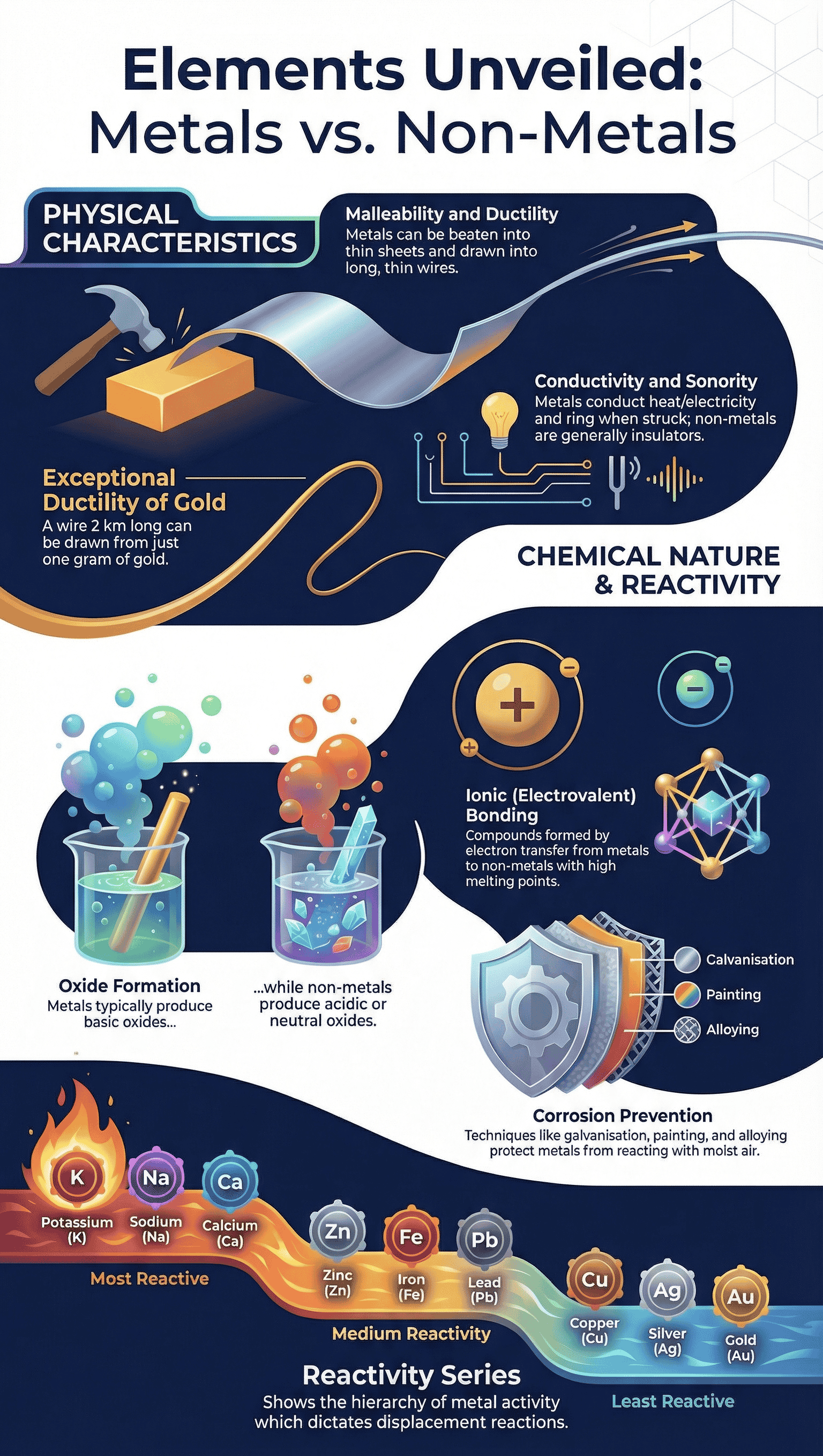
- Metallic Lustre: In their pure state, metals have a shining surface.
- Hardness: Metals are generally hard, though hardness varies (e.g., Iron vs. Sodium).
- Malleability: Metals can be beaten into thin sheets. Gold and silver are the most malleable.
- Ductility: The ability to be drawn into thin wires. Gold is the most ductile metal.
- Conductivity: Metals are good conductors of heat and electricity. Silver and copper are the best thermal conductors; lead and mercury are poor.
- Sonority: Metals produce a ringing sound when striking a hard surface.
Non-metals
- State: Either solids or gases at room temperature (Exception: Bromine is a liquid).
- Properties: generally not lustrous, not sonorous, and are poor conductors of heat and electricity.
- Examples include carbon, sulphur, iodine, oxygen, and hydrogen.
Important Exceptions:
- Mercury: The only metal that is liquid at room temperature.
- Gallium and Caesium: Metals with very low melting points; they melt on a palm.
- Iodine: A non-metal that is lustrous.
- Carbon: Exists as allotropes. Diamond is the hardest natural substance with high melting points. Graphite conducts electricity.
- Alkali Metals (Li, Na, K): Soft enough to be cut with a knife; low densities and melting points.
2. Chemical Properties of Metals
Reaction with Air (Burnt in Oxygen)
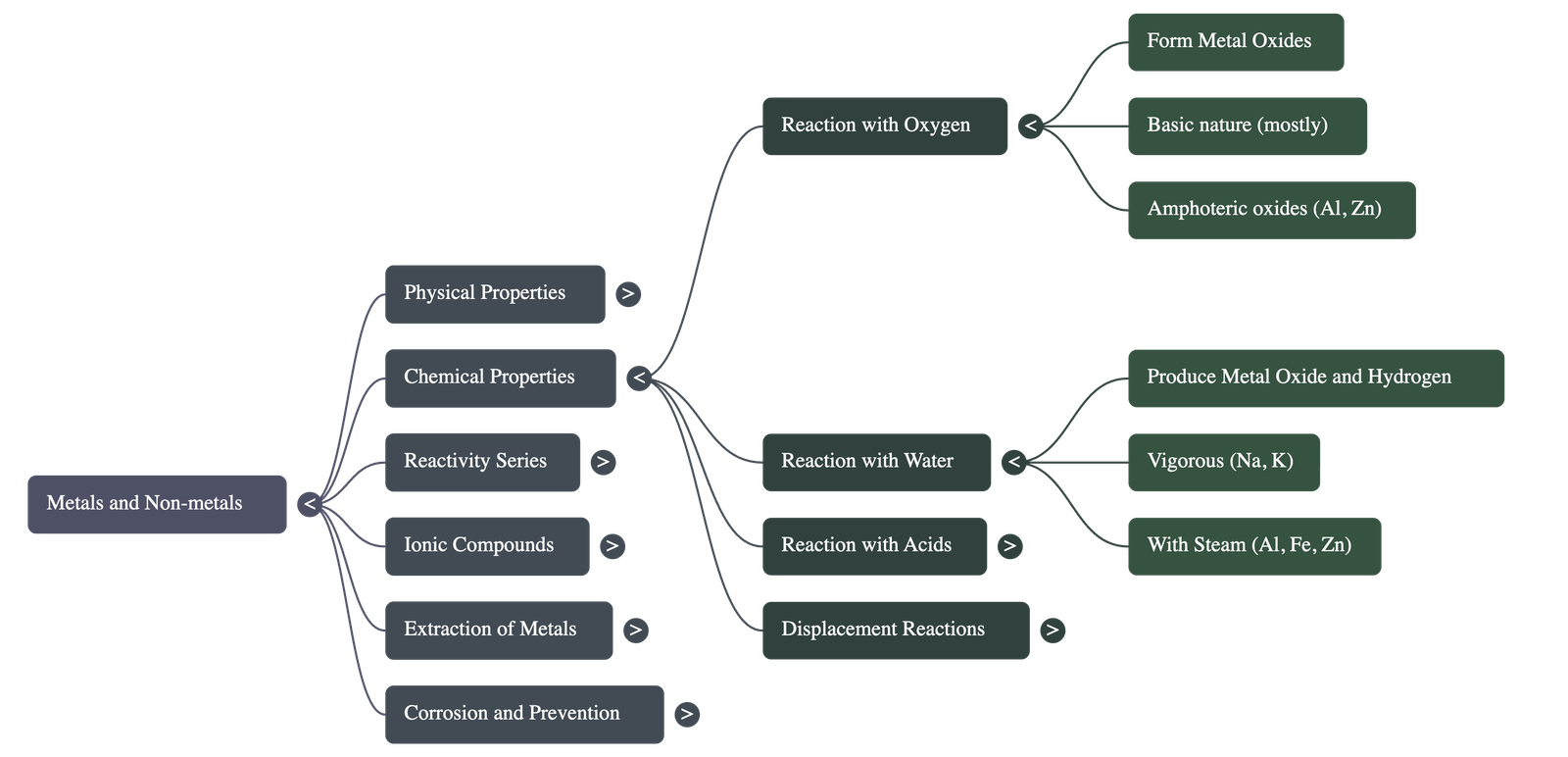
- Almost all metals combine with oxygen to form Metal Oxides.
- Nature of Oxides: Metal oxides are generally basic. However, aluminium oxide and zinc oxide are Amphoteric Oxides (react with both acids and bases to produce salt and water).
- Reactivity varies:
- Sodium and Potassium react so vigorously they catch fire in open air (stored in kerosene).
- Magnesium, Aluminium, Zinc, and Lead form a protective oxide layer that prevents further oxidation.
- Silver and Gold do not react with oxygen even at high temperatures.
- Anodising: A process to form a thick oxide layer on aluminium to improve corrosion resistance.
Reaction with Water
- Metal + Water → Metal Oxide + Hydrogen (Metal oxide may further form Metal Hydroxide).
- Cold Water: Sodium and Potassium react violently (exothermic, hydrogen catches fire). Calcium reacts less violently and floats.
- Hot Water: Magnesium reacts with hot water and floats.
- Steam: Aluminium, Iron, and Zinc do not react with hot/cold water but react with steam to form oxides.
- No Reaction: Lead, Copper, Silver, and Gold do not react with water at all.
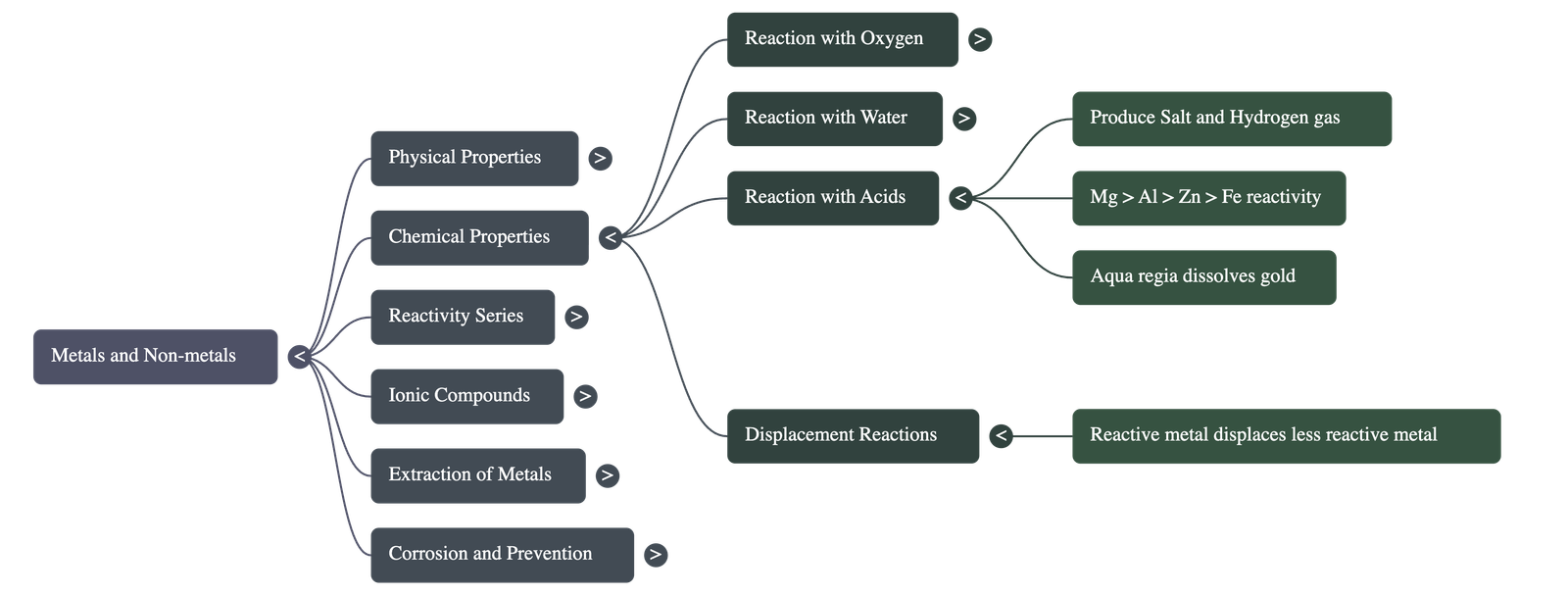
Reaction with Acids
- Metal + Dilute Acid → Salt + Hydrogen.
- Nitric Acid (HNO3): Hydrogen is generally not evolved because HNO3 is a strong oxidising agent (oxidises H2 to water). Exception: Magnesium and Manganese react with very dilute HNO3 to evolve hydrogen.
- Aqua Regia: A mixture of conc. HCl and conc. HNO3 (3:1) capable of dissolving gold and platinum.
Reaction with Other Metal Salts (Displacement)
- Reactive metals can displace less reactive metals from their compounds in solution or molten form.
- Reaction: Metal A + Salt Solution of B → Salt Solution of A + Metal B (if A is more reactive than B).
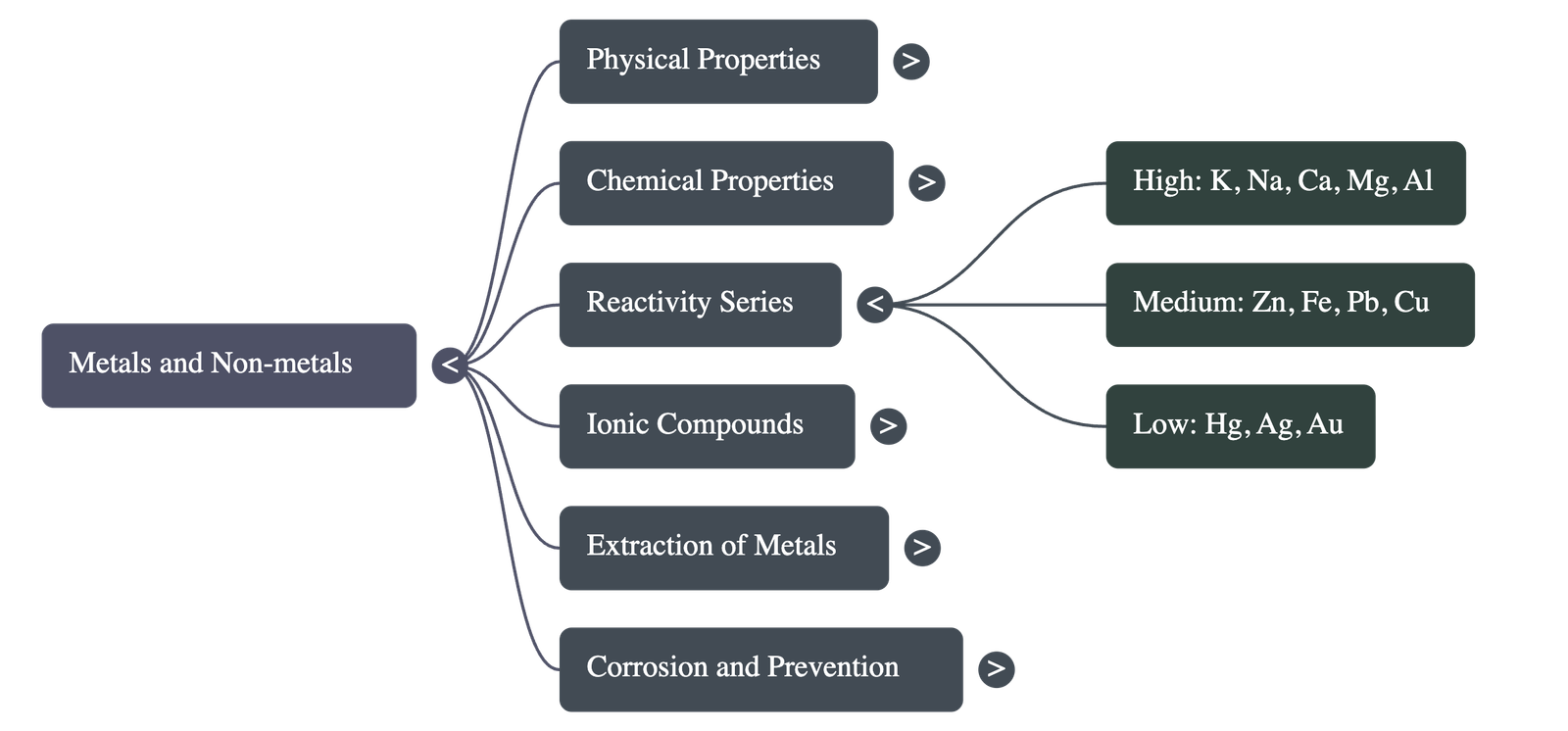
The Reactivity Series
Metals arranged in decreasing order of reactivity:
K > Na > Ca > Mg > Al > Zn > Fe > Pb > [H] > Cu > Hg > Ag > Au
3. How Metals and Non-metals React
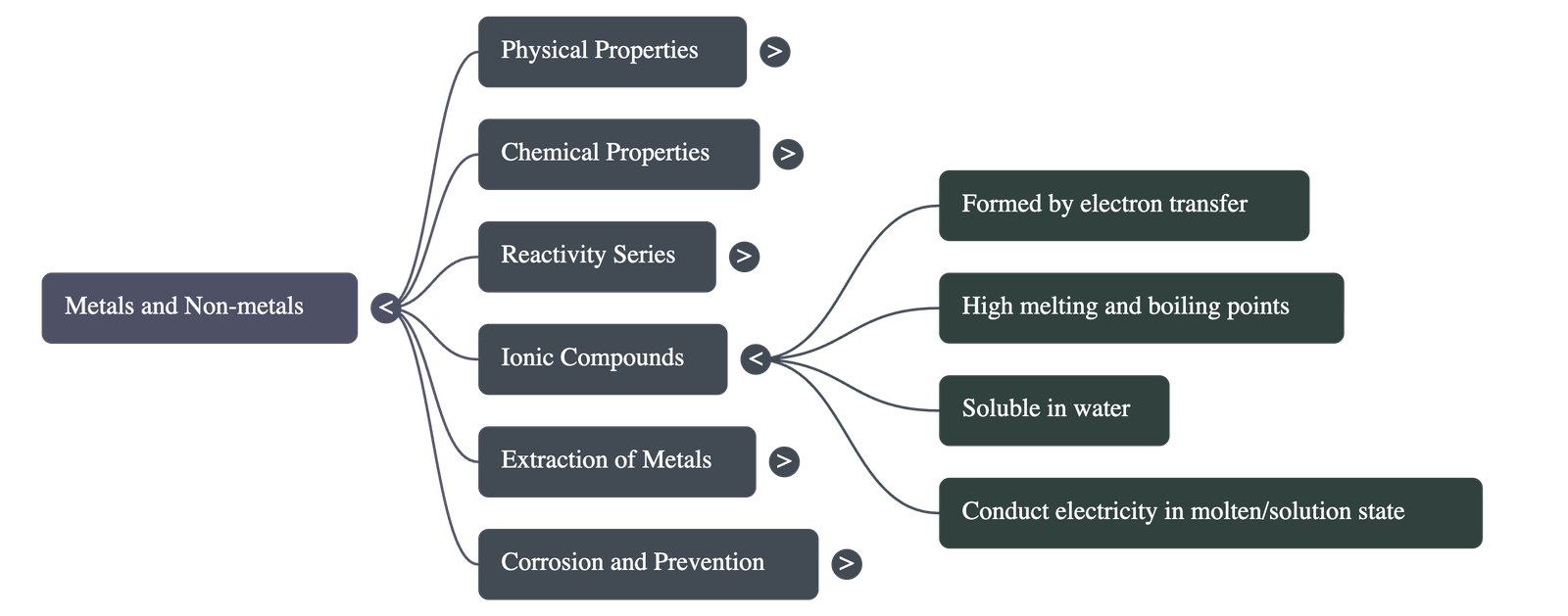
- Electronic Configuration: Elements react to attain a completely filled valence shell (stable octet).
- Ionic Compounds: Formed by the transfer of electrons from a metal (forming a positive cation) to a non-metal (forming a negative anion).
- Properties of Ionic Compounds:
- Physical Nature: Solid and hard due to strong inter-ionic attraction; generally brittle.
- Melting/Boiling Points: High, as considerable energy is needed to break the strong attraction.
- Solubility: Soluble in water; insoluble in solvents like kerosene and petrol.
- Conductivity: Conduct electricity in molten state or aqueous solution (ions move freely), but not in solid state.
4. Occurrence of Metals
- Minerals: Elements/compounds occurring naturally in the earth’s crust.
- Ores: Minerals from which metal can be profitably extracted.
- Gangue: Impurities (soil, sand) present in mined ores.
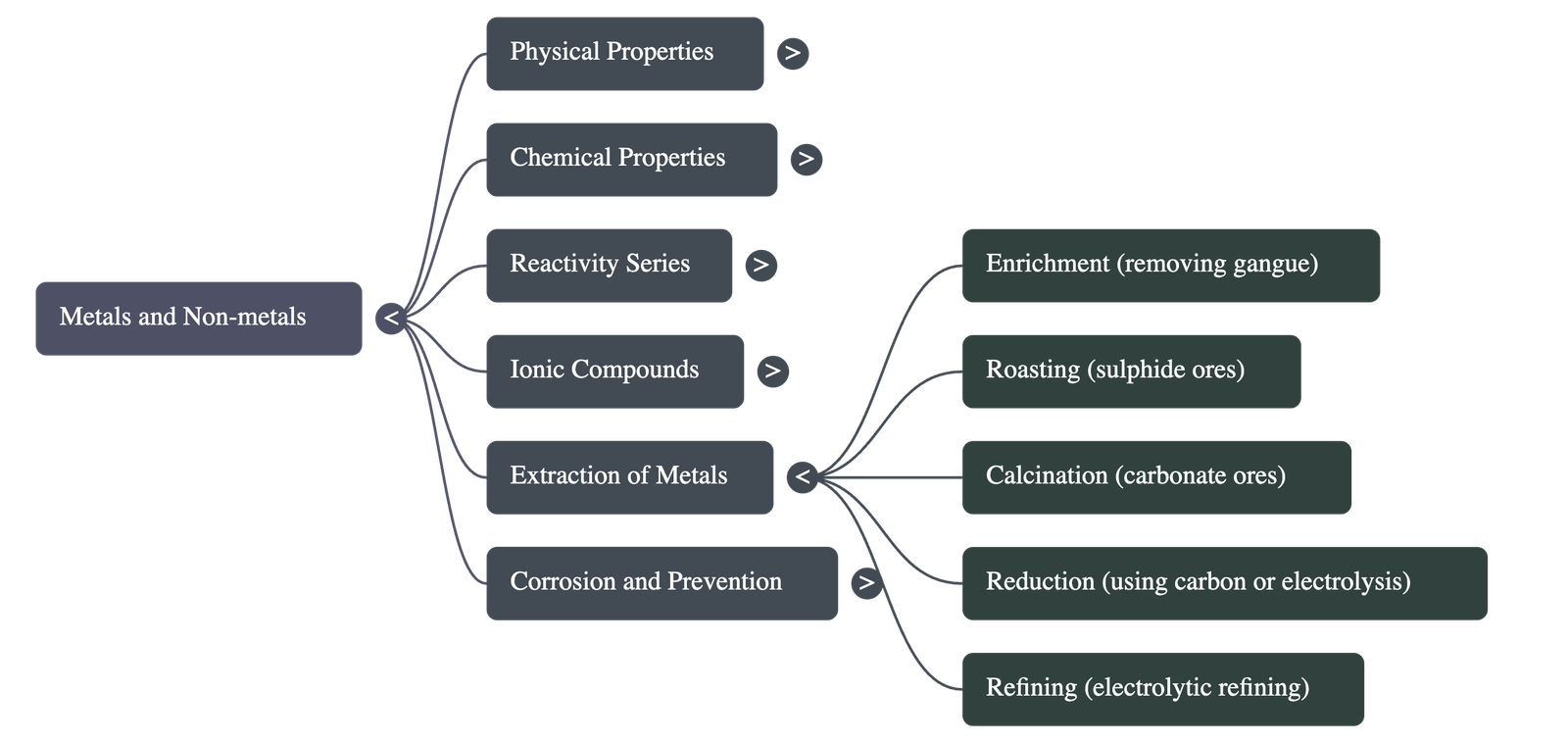
Extraction Based on Reactivity
- Metals low in activity series (e.g., Hg, Cu):
- Oxides can be reduced to metals by heating alone.
- Sulphide ores (like Cinnabar, HgS) are heated in air.
- Metals in the middle of activity series (e.g., Zn, Fe, Pb):
- Usually found as sulphides or carbonates.
- Roasting: Heating sulphide ores in excess air to convert to oxide.
- Calcination: Heating carbonate ores in limited air to convert to oxide.
- Reduction: Metal oxides are reduced using carbon (coke) or highly reactive metals (displacement).
- Thermit Reaction: Reaction of Iron(III) oxide with Aluminium is highly exothermic and used to join railway tracks.
- Metals at the top of activity series (e.g., Na, Mg, Al, Ca):
- Cannot be reduced by carbon due to high affinity for oxygen.
- Obtained by Electrolytic Reduction of their molten chlorides or oxides. Metal deposits at the cathode.
Refining of Metals
- Electrolytic Refining: Used for Cu, Zn, Sn, Ni, Ag, Au.
- Anode: Impure metal. Cathode: Strip of pure metal. Electrolyte: Metal salt solution.
- Pure metal deposits on the cathode; insoluble impurities settle as Anode Mud.
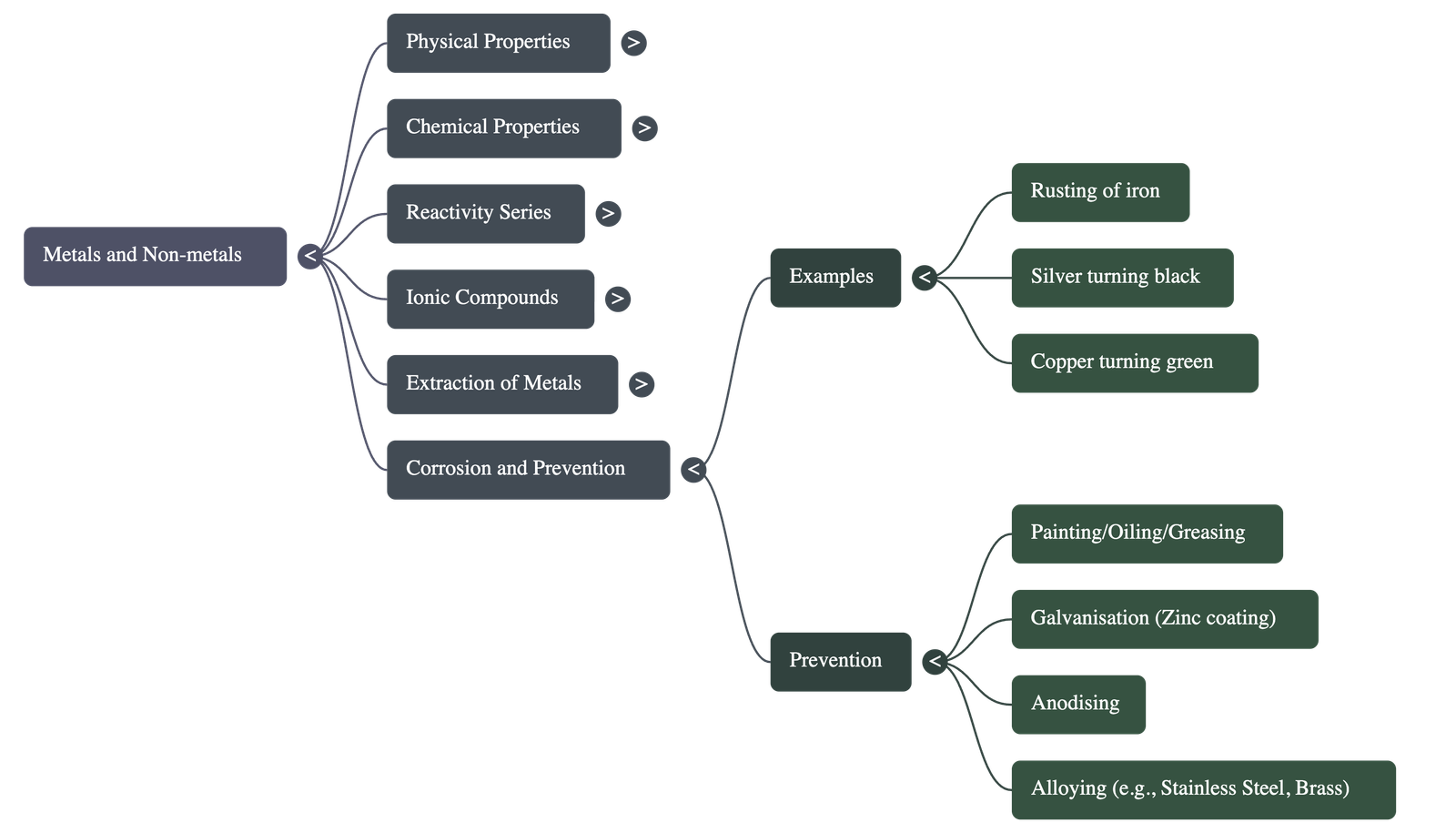
5. Corrosion
- Silver: Turns black due to formation of silver sulphide (reaction with sulphur in air).
- Copper: Turns green due to formation of copper carbonate (reaction with moist CO2).
- Iron: Forms brown flaky rust (hydrated iron oxide) when exposed to moist air. Both air (oxygen) and moisture (water) are required for rusting.
Prevention of Corrosion
- Methods include painting, oiling, greasing, chrome plating, anodising, or making alloys.
- Galvanisation: Coating steel and iron with a thin layer of Zinc.
- Alloying: A homogeneous mixture of two or more metals, or a metal and a non-metal.
- Improves properties (e.g., pure iron is soft; Iron + Carbon is hard).
- Stainless Steel: Iron + Nickel + Chromium (hard, does not rust).
- Brass: Copper + Zinc.
- Bronze: Copper + Tin.
- Solder: Lead + Tin (low melting point, used for welding).
- Amalgam: An alloy where one metal is Mercury.
Quick Navigation:
| | | |
1 / 1
Quick Navigation:
| | | |