Quick Navigation:
| | |
Chapter 4: Structure of the Atom
1. Discovery of Charged Particles
- Introduction: Early experiments with static electricity suggested that atoms are divisible and consist of charged particles.
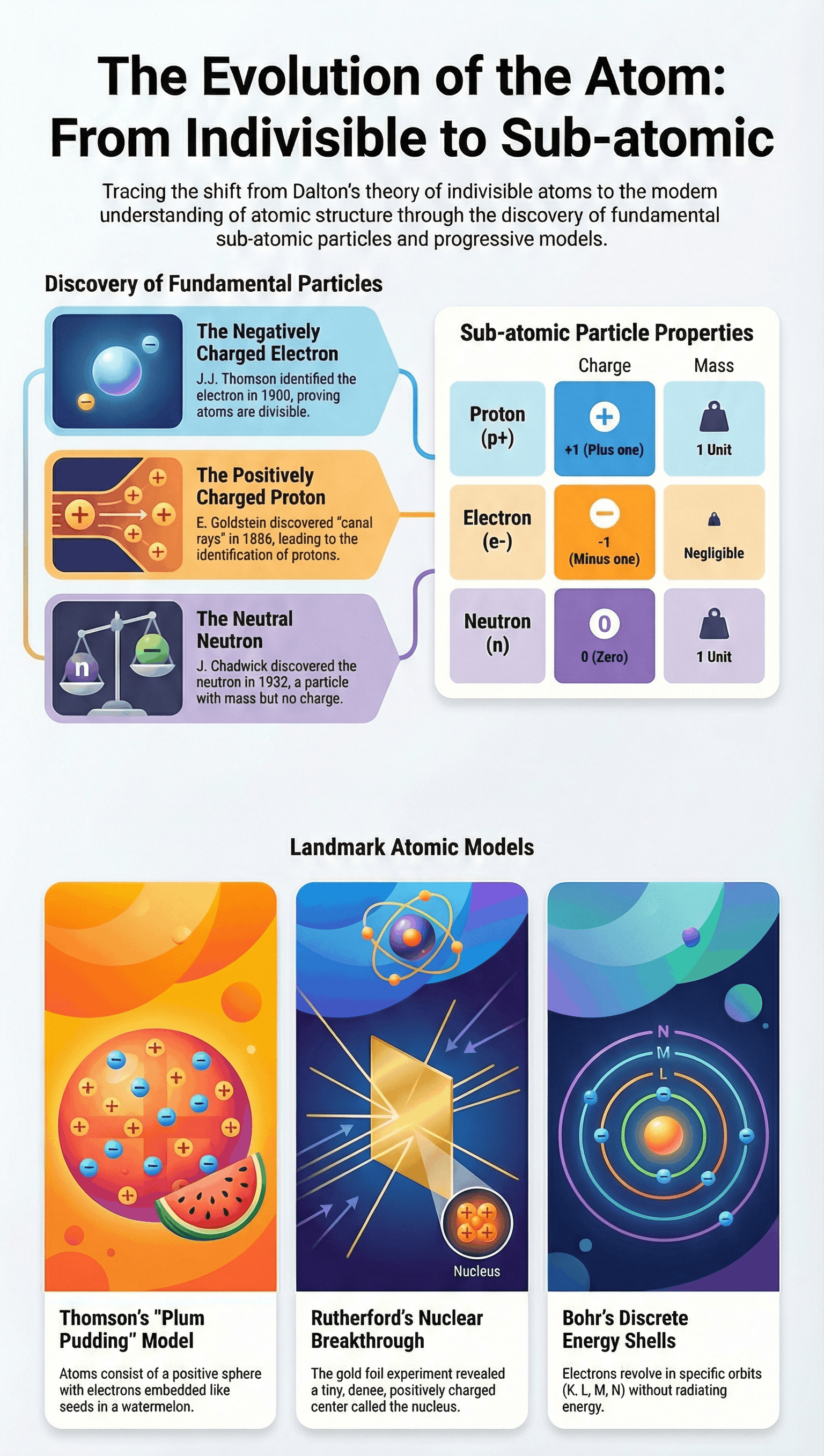
- Canal Rays: Discovered by E. Goldstein in 1886. These were positively charged radiations in a gas discharge which led to the discovery of the proton.
- Electrons: Identified by J.J. Thomson. They are negatively charged sub-atomic particles.
- Protons: Positively charged particles. A proton has a charge equal in magnitude but opposite in sign to that of an electron. Its mass is approximately 2000 times that of an electron.
- Comparison: An electron is represented as e⁻ (charge -1, negligible mass) and a proton as p⁺ (charge +1, mass 1 unit).
2. Atomic Models
A. Thomson’s Model of an Atom
- Comparing an atom to a Christmas pudding or a watermelon.
- The atom is a positively charged sphere with electrons embedded in it (like seeds in a red watermelon).
- The negative and positive charges are equal in magnitude, making the atom electrically neutral.
- Limitation: It could not explain the results of experiments carried out by other scientists (like Rutherford's).
B. Rutherford’s Model of an Atom
The Gold Foil Experiment: Fast-moving alpha particles (doubly-charged helium ions) were bombarded on a thin gold foil.
- Observations:
- Most alpha particles passed straight through the foil.
- Some were deflected by small angles.
- Surprisingly, one out of every 12,000 particles rebounded (deflected by 180°).
- Conclusions:
- Most space inside the atom is empty.
- The positive charge occupies very little space.
- All the positive charge and mass are concentrated in a very small volume called the Nucleus.
- Features of the Nuclear Model: Electrons revolve around the nucleus in circular paths. The nucleus is very small compared to the atom.
- Drawback: Orbital revolution of a charged particle is not stable. The electron would undergo acceleration, radiate energy, and eventually fall into the nucleus, making matter unstable.
C. Bohr’s Model of an Atom
- Proposed by Neils Bohr to overcome objections to Rutherford’s model.
- Only certain special orbits known as discrete orbits of electrons are allowed inside the atom.
- While revolving in these discrete orbits, electrons do not radiate energy.
- These orbits are called energy levels or shells, represented by letters K, L, M, N... or numbers n=1, 2, 3, 4...
3. Neutrons
- Discovered by J. Chadwick in 1932.
- A sub-atomic particle with no charge and a mass nearly equal to that of a proton.
- Present in the nucleus of all atoms except Hydrogen.
- The mass of an atom is the sum of the masses of protons and neutrons in the nucleus.
4. Distribution of Electrons and Valency
- Bohr-Bury Scheme: Rules for filling electrons in shells.
- Maximum Electrons: The maximum number of electrons in a shell is given by the formula 2n² (where 'n' is the orbit number).
Examples: K-shell (n=1) = 2; L-shell (n=2) = 8; M-shell (n=3) = 18. - Outer Shell Limit: The maximum number of electrons that can be accommodated in the outermost orbit is 8.
- Filling Order: Inner shells must be filled before outer shells (step-wise manner).
- Valency: The combining capacity of an atom. It is determined by the number of valence electrons (electrons in the outermost shell).
- Atoms with 8 electrons in the outer shell have an octet and are chemically inert (zero valency).
- Atoms react to achieve a fully filled outer shell by losing, gaining, or sharing electrons.
- Calculation: If outer electrons ≤ 4, Valency = number of electrons. If outer electrons > 4, Valency = 8 minus number of electrons.
5. Atomic Number and Mass Number
- Atomic Number (Z): The total number of protons present in the nucleus of an atom. Elements are defined by their atomic number.
- Mass Number (A): The sum of the total number of protons and neutrons present in the nucleus. Protons and neutrons are collectively called nucleons.
- Notation: An atom is represented with Mass Number (A) as superscript and Atomic Number (Z) as subscript (Example: ¹⁴N₇).
6. Isotopes and Isobars
- Isotopes: Atoms of the same element having the same atomic number but different mass numbers (same protons, different neutrons).
Examples: Hydrogen has three isotopes: Protium, Deuterium, and Tritium. Carbon has ¹²C and ¹⁴C. Chlorine has ³⁵Cl and ³⁷Cl. - Average Atomic Mass: For elements with isotopes, the atomic mass is the weighted average of the masses of the naturally occurring isotopes. (e.g., Chlorine is 35.5 u).
- Applications of Isotopes:
- Uranium isotope: Fuel in nuclear reactors.
- Cobalt isotope: Treatment of cancer.
- Iodine isotope: Treatment of goitre.
- Isobars: Atoms of different elements with different atomic numbers that have the same mass number (total nucleons are the same).
Example: Calcium (Atomic Number 20) and Argon (Atomic Number 18) both have a Mass Number of 40.
Summary based on NCERT Science Chapter 4.
Quick Navigation:
| | |
1 / 1
Quick Navigation:
| | |