Quick Navigation:
| | |
Matter in Our Surroundings
1. Introduction to Matter
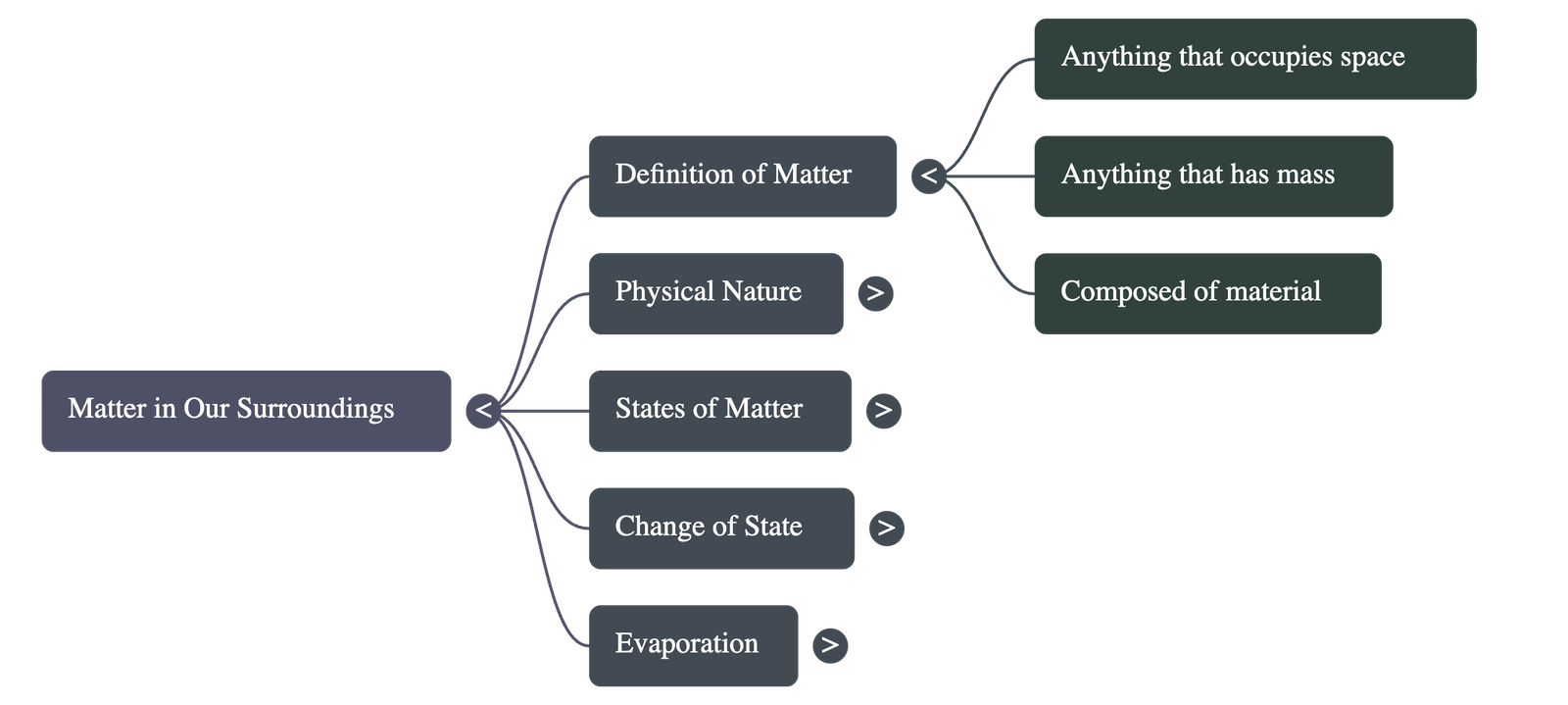
- Definition: Matter is anything that occupies space (volume) and has mass.
- Examples: Air, food, stones, clouds, stars, plants, animals, and water droplets.
- Ancient Classification (Panch Tatva): Early Indian philosophers classified matter into five basic elements: Air, Earth, Fire, Sky, and Water.
- Modern Classification: Scientists classify matter based on physical properties and chemical nature. This chapter focuses on physical properties.
2. Physical Nature of Matter
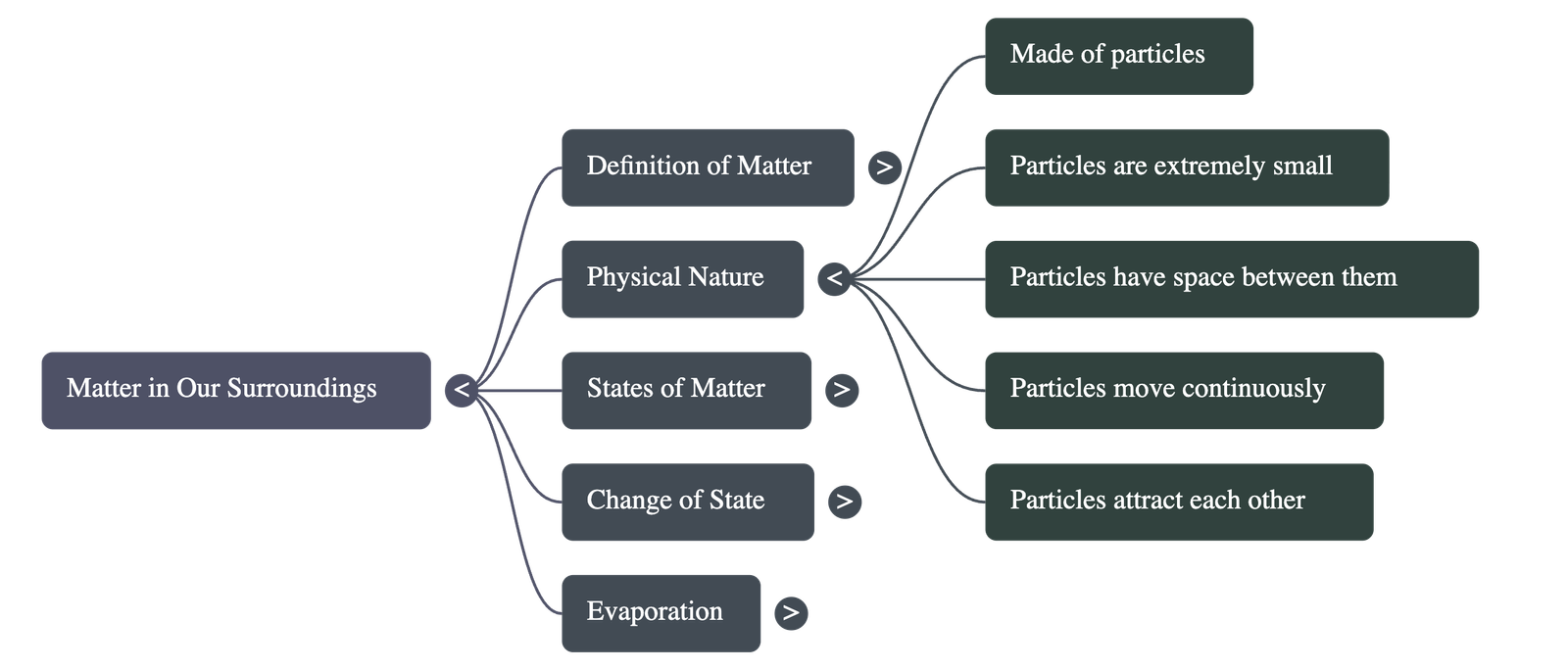
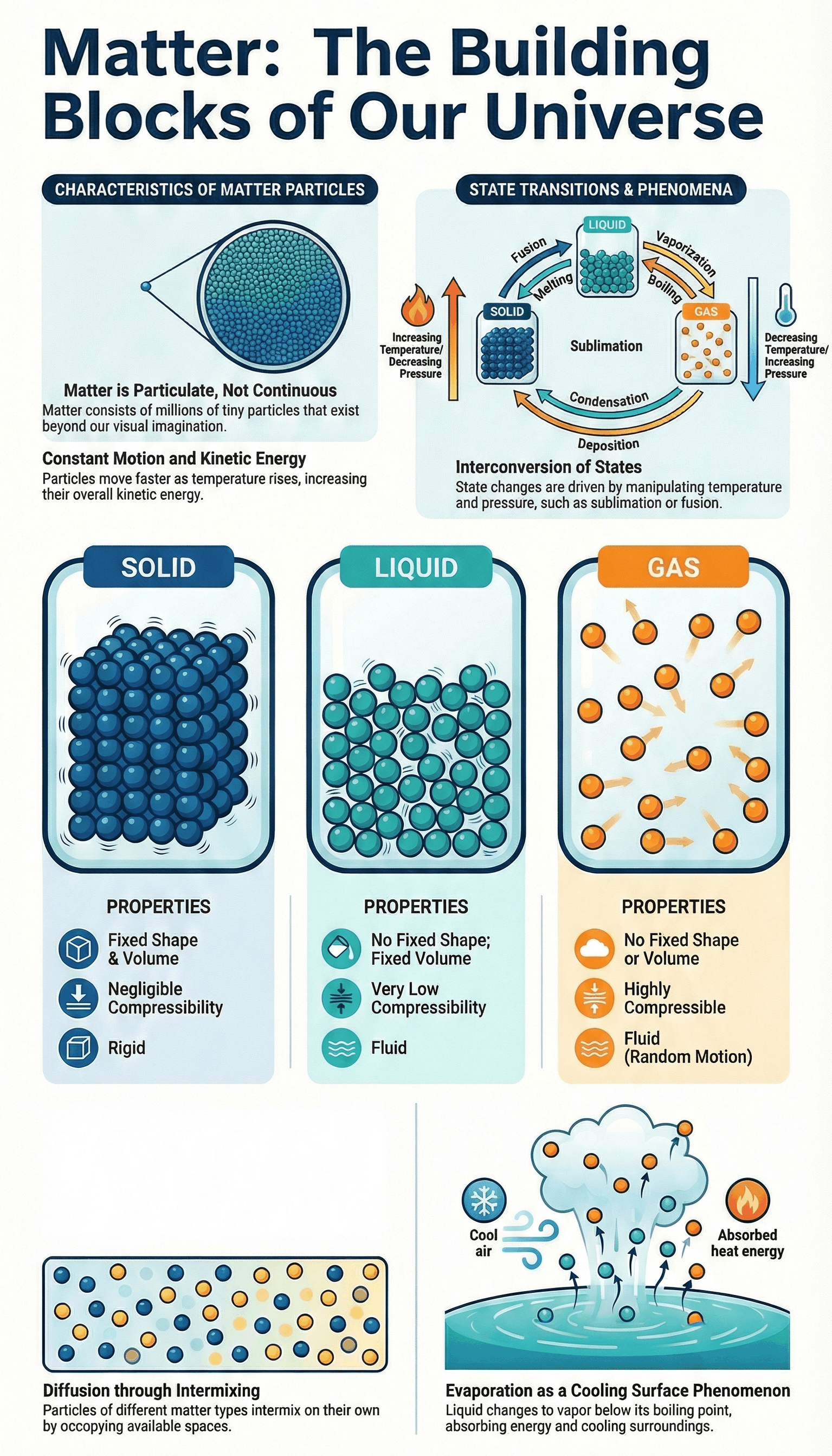
- Particulate Nature: Matter is not continuous (like a block of wood) but is made up of particles (like sand).
- Extremely Small Size: Particles are small beyond imagination. For example, a few crystals of potassium permanganate or a few milliliters of Dettol can colour or scent a large volume of water (1000 L), showing that one crystal contains millions of tiny particles.
3. Characteristics of Particles of Matter
A. Space Between Particles
- Particles have space between them.
- Evidence: When dissolving salt, sugar, or potassium permanganate in water, the particles of the solute get into the spaces between the particles of water.
B. Continuous Motion
- Particles are continuously moving and possess Kinetic Energy.
- Temperature Effect: As temperature rises, particles move faster because their kinetic energy increases.
- Diffusion: The intermixing of particles of two different types of matter on their own is called diffusion. Heating accelerates diffusion.
C. Attraction Between Particles
- Particles attract each other; this force keeps them together.
- The strength of this force varies: it is strongest in solids (e.g., iron nail), intermediate in liquids (e.g., water), and weakest in gases (e.g., air).
4. The States of Matter
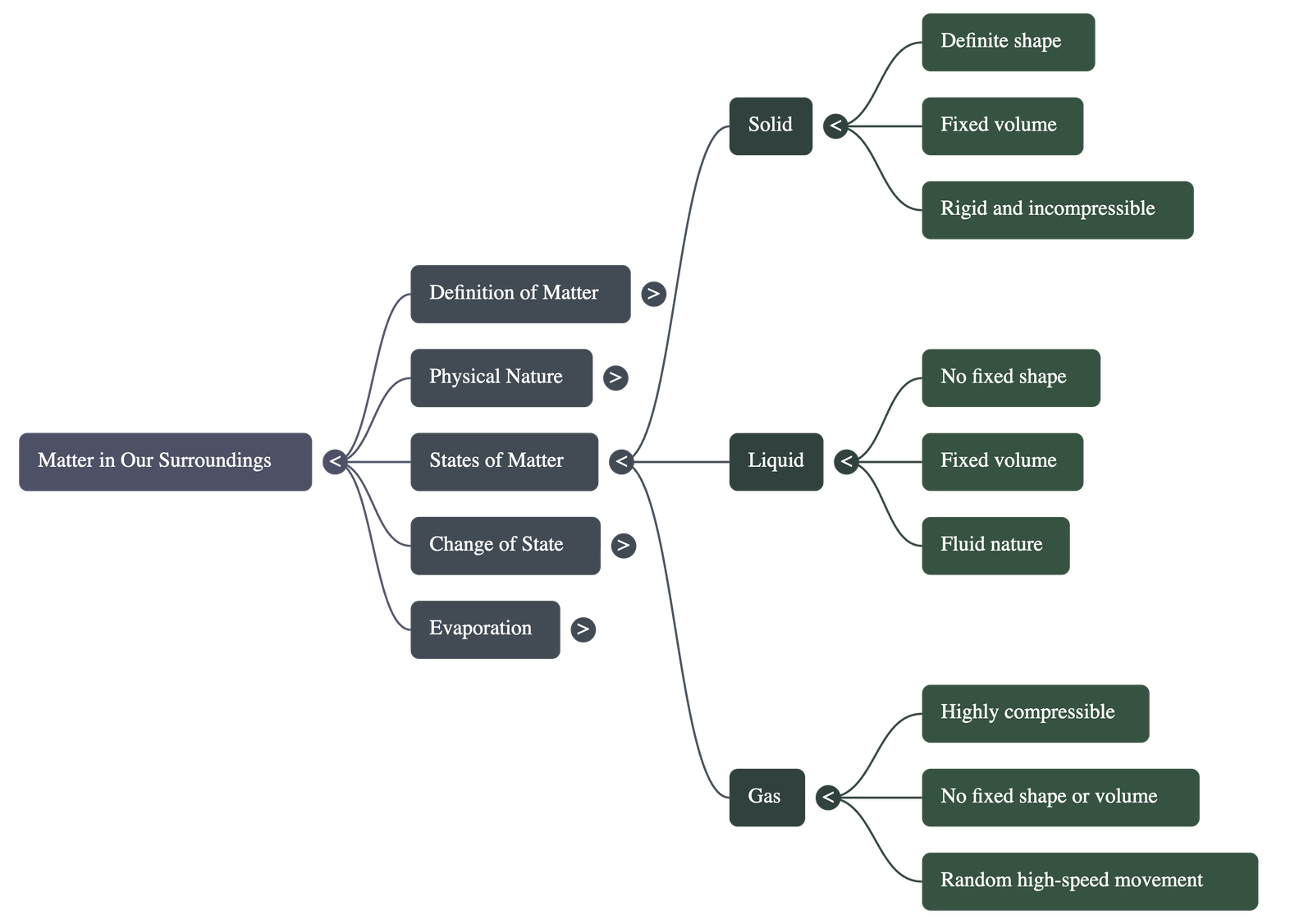
The Solid State
- Definite shape, distinct boundaries, and fixed volume.
- Rigid: Maintains shape when subjected to outside force.
- Negligible compressibility.
- Note: Sponges are solids but compressible because air is trapped in minute holes. Rubber bands change shape under force but regain it when the force is removed.
The Liquid State
- No fixed shape but has a fixed volume. Takes the shape of the container.
- Fluid: Flows and changes shape; not rigid.
- Liquids have more space between particles than solids and allow diffusion of solids, liquids, and gases (e.g., aquatic animals breathe dissolved oxygen).
The Gaseous State
- No fixed shape or volume.
- Highly Compressible: Large volumes can be compressed into small cylinders (e.g., LPG, CNG, Oxygen cylinders).
- Particles move randomly at high speed, exerting pressure on container walls.
- Very fast rate of diffusion.
5. Change of State
Effect of Temperature
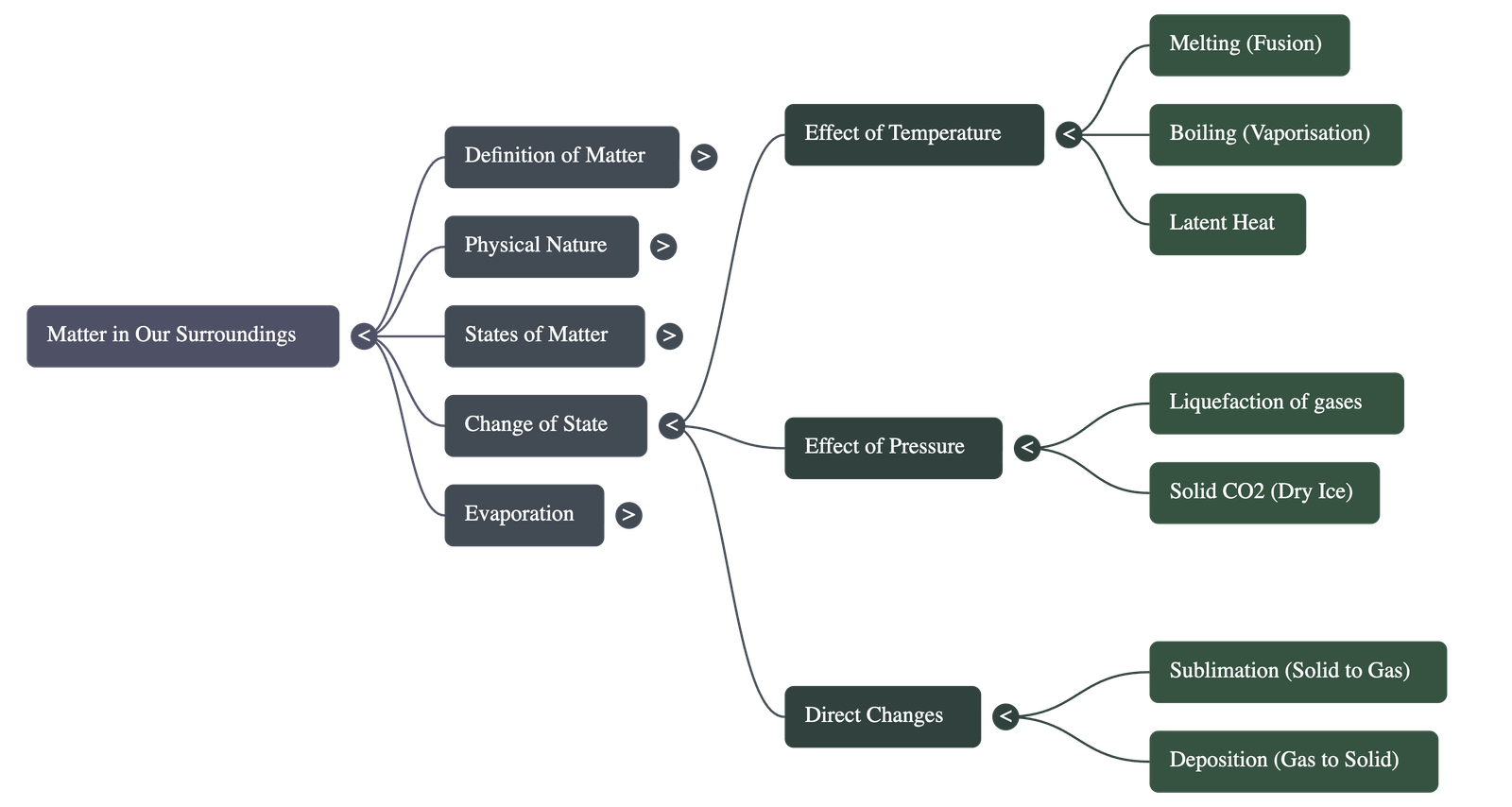
- Solid to Liquid (Melting): Heat overcomes attraction forces.
- Melting Point: Temperature at which solid melts to liquid at atmospheric pressure. Indicates strength of particle attraction.
- Latent Heat of Fusion: Heat energy required to change 1 kg of solid to liquid at melting point. Temp remains constant during melting as heat is used to change state.
- Liquid to Gas (Boiling): Particles gain enough energy to break free.
- Boiling Point: Temperature at which liquid starts boiling. It is a bulk phenomenon.
- Latent Heat of Vaporisation: Heat energy required to change 1 kg of liquid to gas at atmospheric pressure at boiling point.
- Sublimation: Direct change from solid to gas without becoming liquid (e.g., Camphor, Ammonium chloride).
- Deposition: Direct change from gas to solid.
Temperature Unit Conversion:
SI unit is Kelvin (K).
0°C = 273 K (approx).
Kelvin = Celsius + 273.
Celsius = Kelvin - 273.
SI unit is Kelvin (K).
0°C = 273 K (approx).
Kelvin = Celsius + 273.
Celsius = Kelvin - 273.
Effect of Pressure
- Applying pressure and lowering temperature can liquefy gases.
- Dry Ice: Solid Carbon Dioxide (CO2). Converts directly to gas when pressure is reduced to 1 atmosphere.
6. Evaporation
- Definition: Change of liquid into vapour at any temperature below its boiling point.
- It is a surface phenomenon.
Factors Affecting Evaporation
- Surface Area: Increase in area increases rate (e.g., spreading clothes).
- Temperature: Increase in temp increases rate (more kinetic energy).
- Humidity: Increase in humidity decreases rate.
- Wind Speed: Increase in wind speed increases rate.
Cooling Effect
- Evaporation causes cooling because particles absorb energy from surroundings to regain lost energy.
- Examples: Acetone on palm, water in earthen pot (matka), sprinkling water on hot roofs, wearing cotton in summer (absorbs sweat for evaporation).
7. Key Quantities and Units
| Quantity | Unit | Symbol |
|---|---|---|
| Temperature | Kelvin | K |
| Mass | Kilogram | kg |
| Weight | Newton | N |
| Volume | Cubic metre | m³ |
| Density | Kg per cubic metre | kg m⁻³ |
| Pressure | Pascal | Pa |
Quick Navigation:
| | |
1 / 1
Quick Navigation:
| | |