Chapter 2: IS MATTER AROUND US PURE?
1. Introduction to Purity and Mixtures
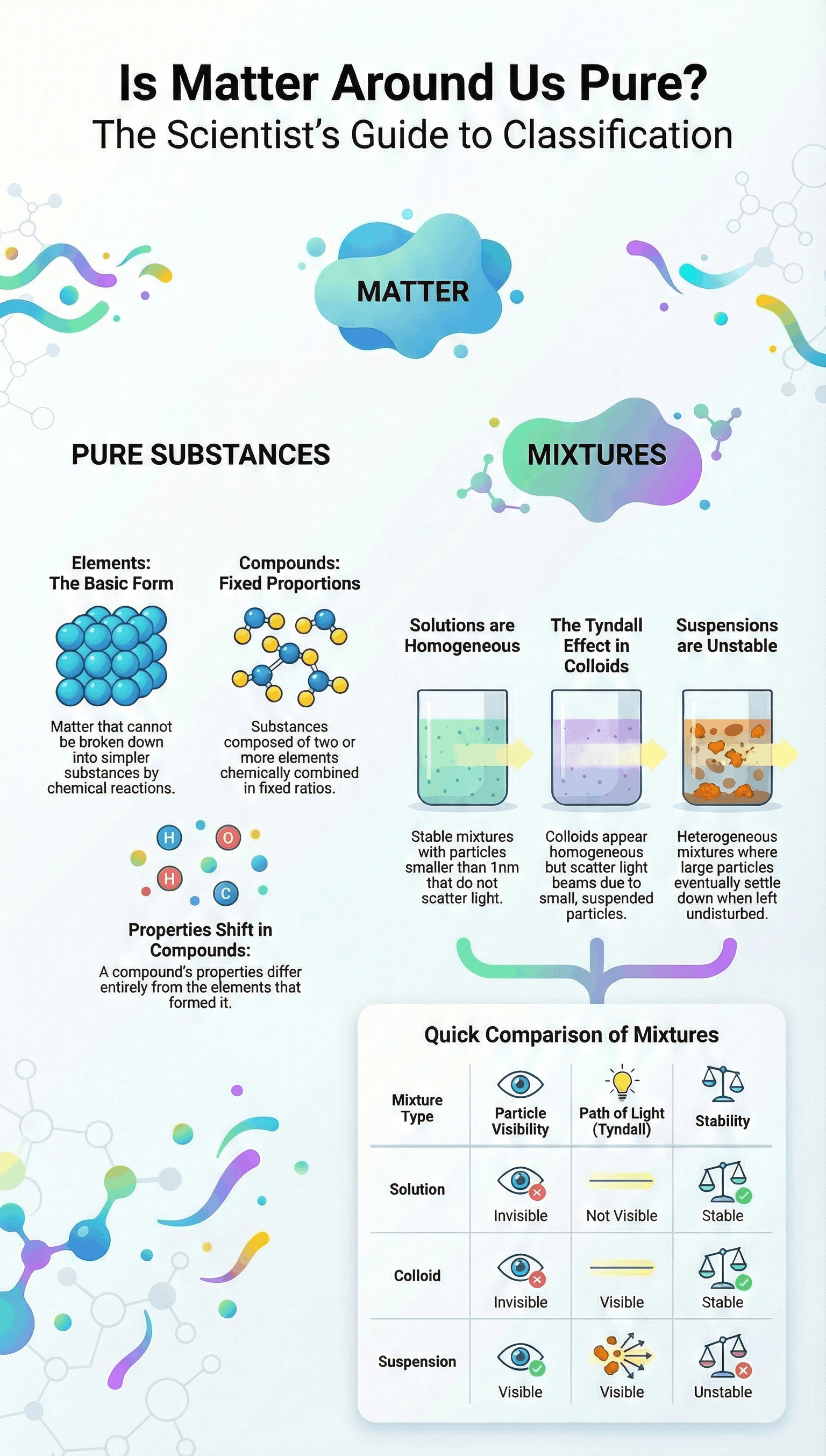
- Scientific Definition of Pure: To a common person, "pure" means no adulteration. To a scientist, it means all constituent particles of a substance are the same in their chemical nature. A pure substance consists of a single type of particle.
- Mixtures: Most matter around us (like sea water, minerals, soil, milk) exists as mixtures. Mixtures are constituted by more than one kind of pure form of matter (substance).
- Separation: A substance cannot be separated into other kinds of matter by any physical process (e.g., dissolved sodium chloride can be separated from water by evaporation, but sodium chloride itself is a pure substance).
2. Types of Mixtures
Depending on the nature of the components, mixtures are classified into two main types:
- Homogeneous Mixtures (Solutions): Mixtures with a uniform composition throughout.
Examples: Salt dissolved in water, sugar in water. - Heterogeneous Mixtures: Mixtures containing physically distinct parts and non-uniform composition.
Examples: Sodium chloride and iron filings, oil and water, salt and sulphur.
3. What is a Solution?
A solution is a homogeneous mixture of two or more substances. Homogeneity exists at the particle level.
Components of a Solution
- Solvent: The component that dissolves the other component (usually present in larger amount).
- Solute: The component dissolved in the solvent (usually present in lesser quantity).
- Examples: Tincture of iodine (iodine in alcohol), aerated drinks (CO2 in water), air (gas in gas).
Properties of a Solution
- It is a homogeneous mixture.
- Particles are extremely small (less than 1 nm in diameter) and cannot be seen by naked eyes.
- They do not scatter a beam of light (path of light is not visible).
- The solution is stable; solute particles do not settle when undisturbed.
- Filtration cannot separate the solute particles.
Concentration of a Solution
The concentration is the amount of solute present in a given amount of solution.
- Saturated Solution: A solution in which no more solute can be dissolved at a given temperature.
- Unsaturated Solution: If the amount of solute is less than the saturation level.
- Solubility: The amount of solute present in a saturated solution at a specific temperature.
Formulas for Concentration:
2. Mass by volume percentage = (Mass of solute / Volume of solution) × 100
4. Suspensions
A suspension is a heterogeneous mixture containing solids dispersed in liquids where the solute particles do not dissolve but remain suspended.
Properties of a Suspension
- It is a heterogeneous mixture.
- Particles are visible to the naked eye.
- Particles scatter a beam of light making the path visible (until they settle).
- It is unstable; particles settle down when left undisturbed.
- Components can be separated by filtration.
5. Colloidal Solutions
A colloid appears homogeneous but is actually heterogeneous (e.g., milk). The particle size is between that of a true solution and a suspension.
Properties of a Colloid
- It is a heterogeneous mixture.
- Particles are too small to be seen individually by the naked eye.
- Tyndall Effect: Colloids are big enough to scatter a beam of light passing through them, making the path visible (e.g., sunlight through a canopy of a dense forest).
- Colloids are stable (particles do not settle).
- Cannot be separated by filtration but can be separated by centrifugation.
Components
- Dispersed Phase: The solute-like component.
- Dispersion Medium: The component in which the dispersed phase is suspended.
- Examples: Fog (liquid in gas), Smoke (solid in gas), Shaving cream (gas in liquid), Milk (liquid in liquid).
6. Physical and Chemical Changes
-
Physical Change: Changes that occur without a change in composition or chemical nature.
Characteristics: Interconversion of states (solid to liquid to gas). Although ice, water, and steam look different, they are chemically the same. -
Chemical Change: One substance reacts with another to undergo a change in chemical composition.
Characteristics: Brings change in chemical properties and results in new substances. Also called a chemical reaction.
Example: Burning (change in odour and inflammability).
7. Types of Pure Substances
Pure substances are classified into elements and compounds based on chemical composition.
Elements
An element is a basic form of matter that cannot be broken down into simpler substances by chemical reactions (Definition by Lavoisier). They are divided into:
- Metals: Lustrous, conduct heat/electricity, ductile, malleable, sonorous (e.g., Gold, Iron, Copper). Mercury is the only liquid metal at room temperature.
- Non-metals: Non-lustrous, poor conductors, not malleable/sonorous, variety of colours (e.g., Oxygen, Carbon, Iodine).
- Metalloids: Intermediate properties between metals and non-metals (e.g., Boron, Silicon).
Compounds
A compound is a substance composed of two or more elements chemically combined in a fixed proportion.
Key distinction from mixtures: When elements are just mixed (e.g., iron filings and sulphur powder), they retain their individual properties (magnetism). When heated strongly to react, they form a compound with entirely new properties (non-magnetic).
8. Comparison: Mixtures vs. Compounds
| Mixtures | Compounds |
|---|---|
| Elements or compounds just mix together; no new compound formed. | Elements react to form new compounds. |
| Variable composition. | Composition of each new substance is always fixed. |
| Shows the properties of the constituent substances. | The new substance has totally different properties. |
| Constituents can be separated fairly easily by physical methods. | Constituents separated only by chemical or electrochemical reactions. |