Quick Navigation:
| | | | |
Quick Navigation:
| | | | |
Water
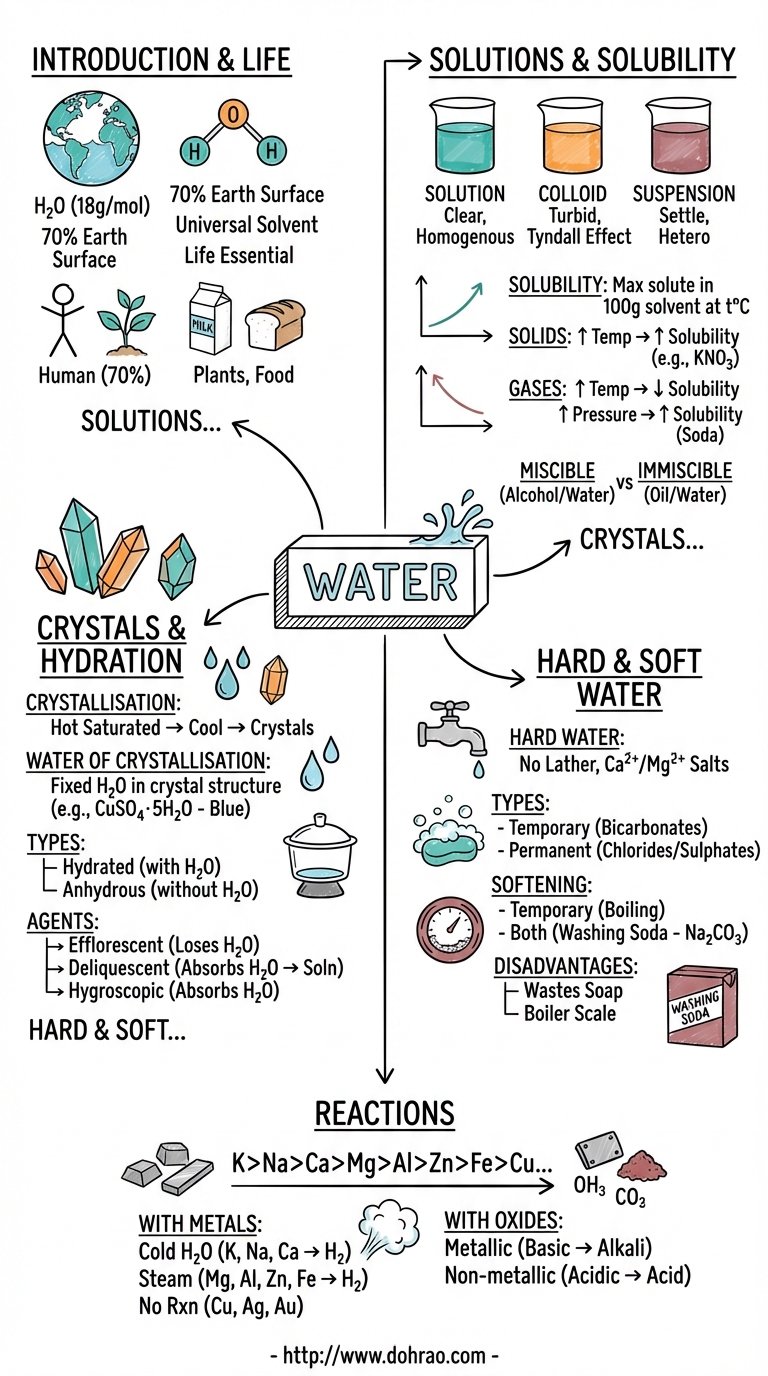
Introduction and Occurrence
- Water is a vital compound of hydrogen and oxygen with the molecular formula H2O and a molecular weight of 18.
- It covers approximately 70% of the Earth's surface and is found in both free and combined states.
- Natural water exists in various forms: rain water is the purest form, while sea water is the most impure due to dissolved salts like sodium chloride.
- It is a primary constituent of all living things, making up 70% of the human body, 85% of milk, and 74% of tomatoes.
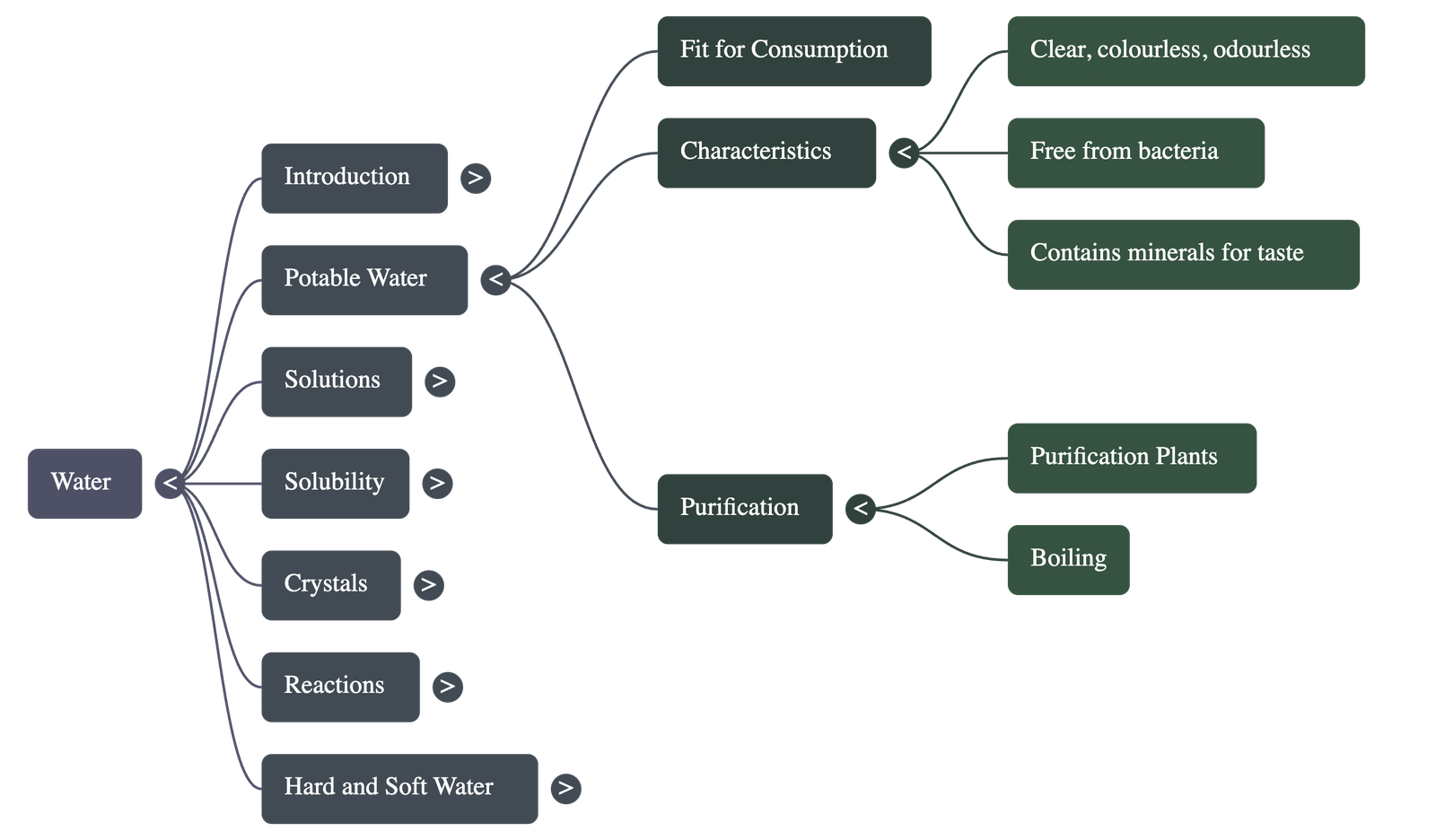
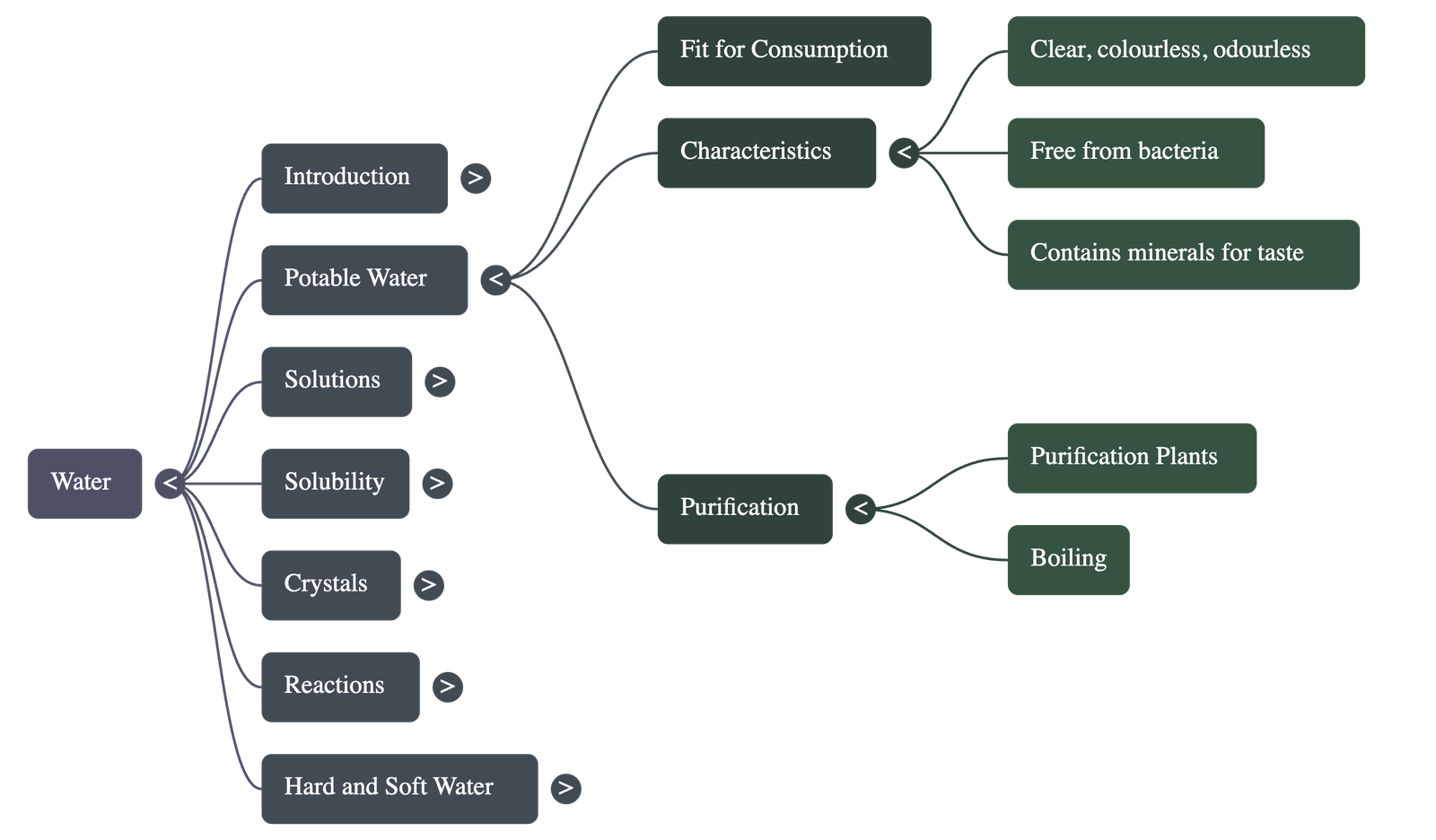
Potable Water and Purification
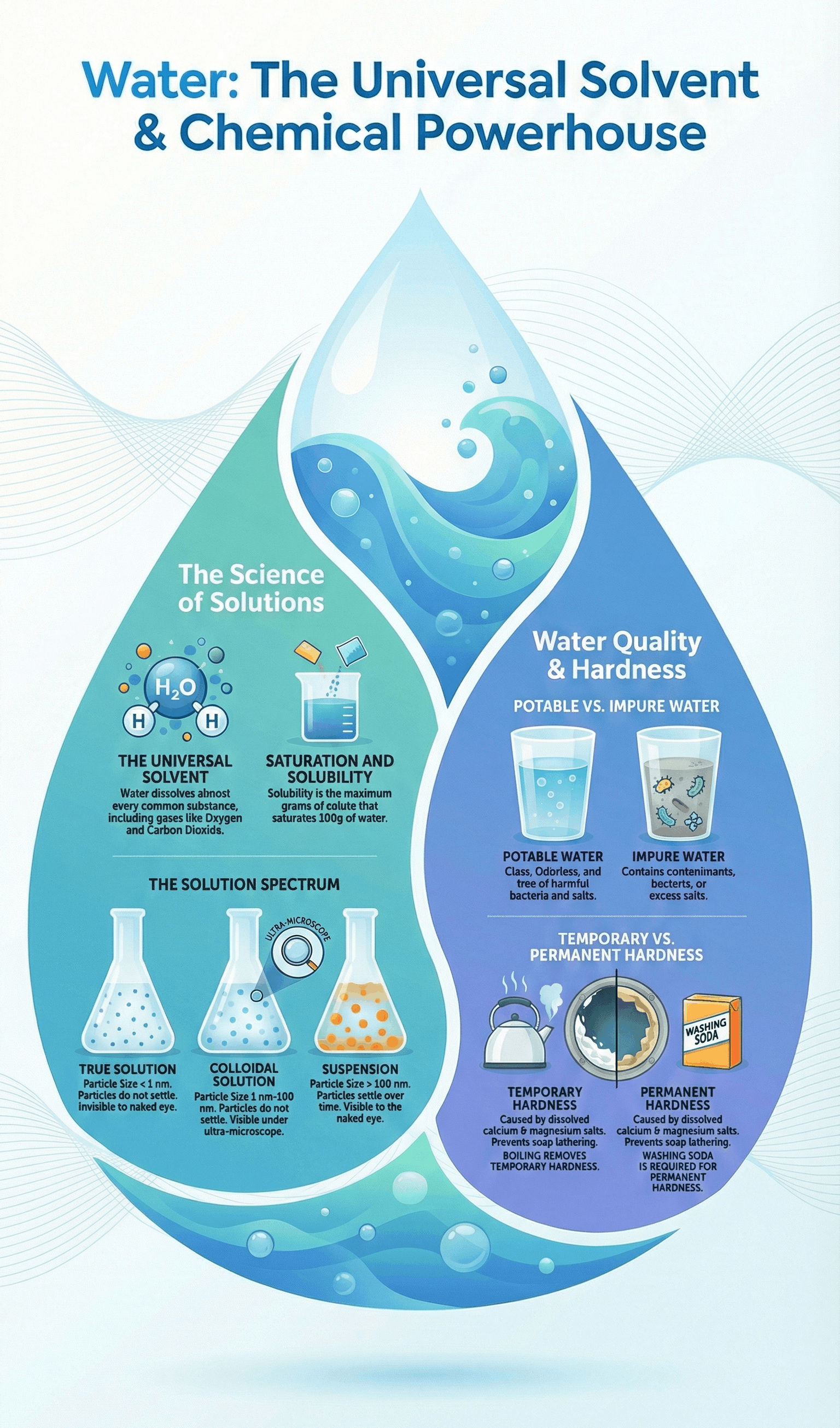
- Potable water is water fit for human consumption; it must be clear, odorless, colorless, and free from harmful bacteria or salts like nitrates and cyanides.
- It should contain small amounts of dissolved gases and minerals that provide taste and aid metabolic functions.
- Water can be unfit for consumption if it is saline (too much salt) or distilled (lacks essential minerals, which can lead to deficiencies).
- Purification methods include using large-scale treatment plants for cities or boiling for small quantities to kill germs.
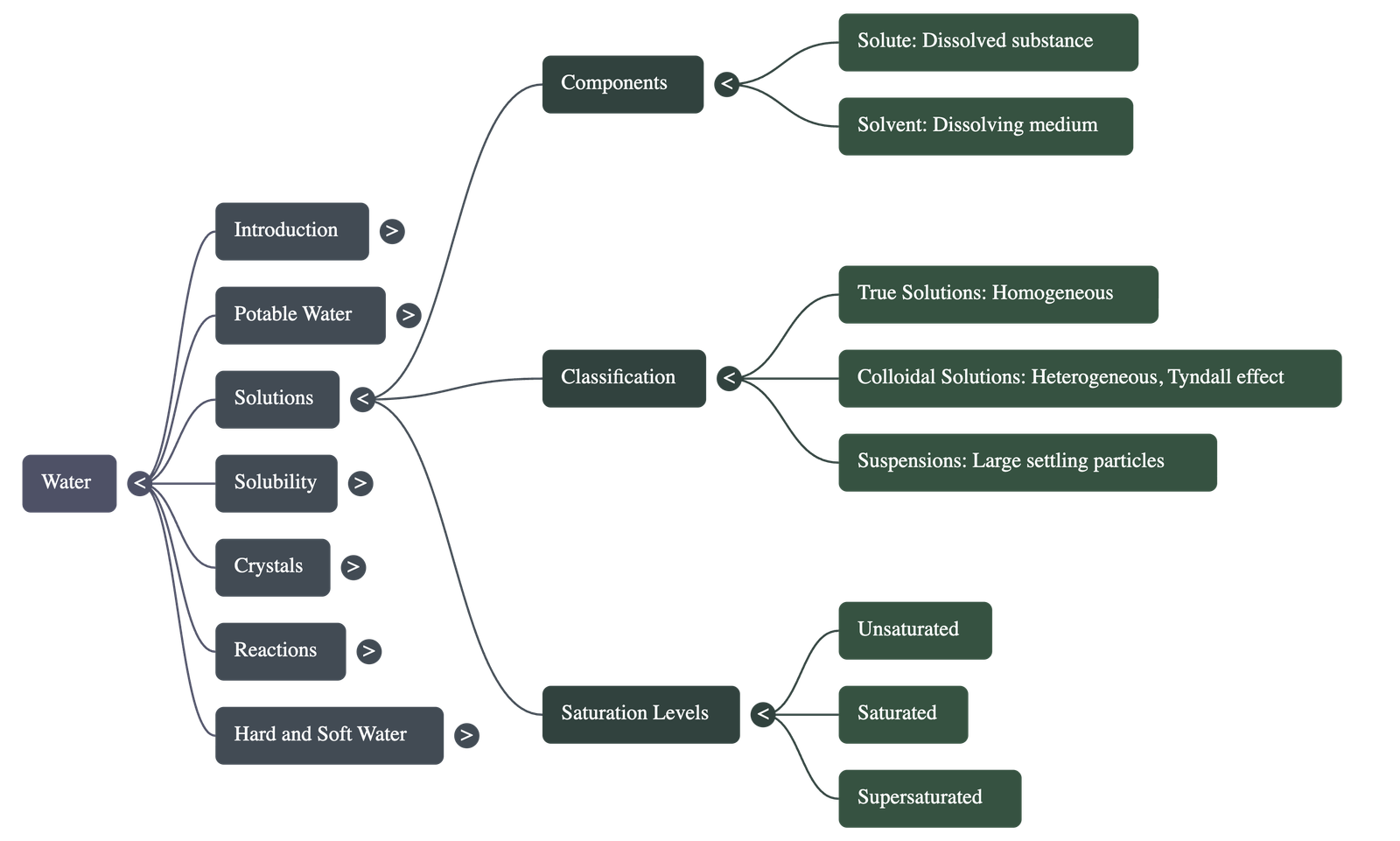
Water as a Universal Solvent
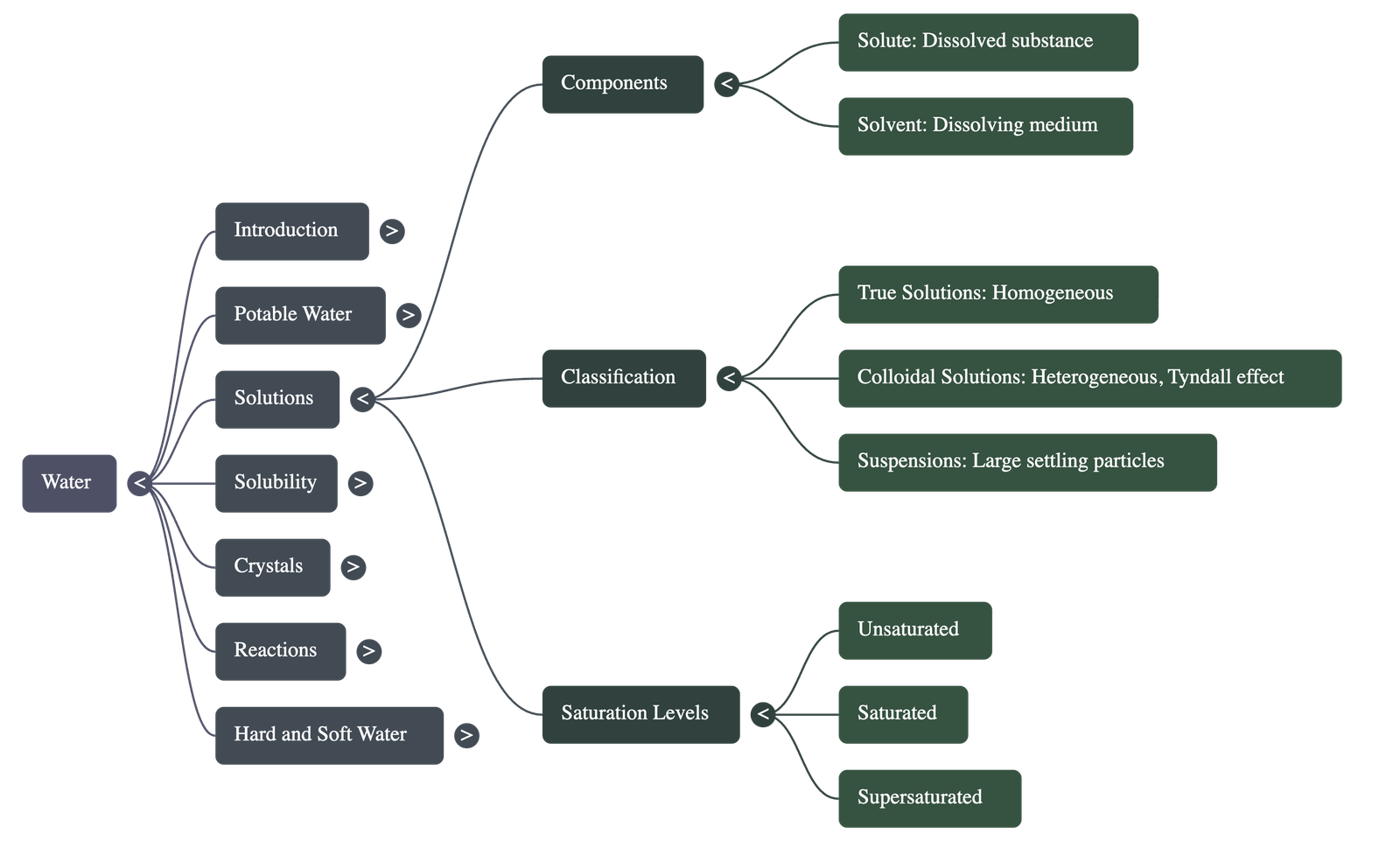
- Water is known as the universal solvent because it can dissolve almost every common substance, including various solids and gases.
- A solute is the substance that dissolves (e.g., salt), a solvent is the medium of dissolution (e.g., water), and a solution is the resulting homogeneous mixture.
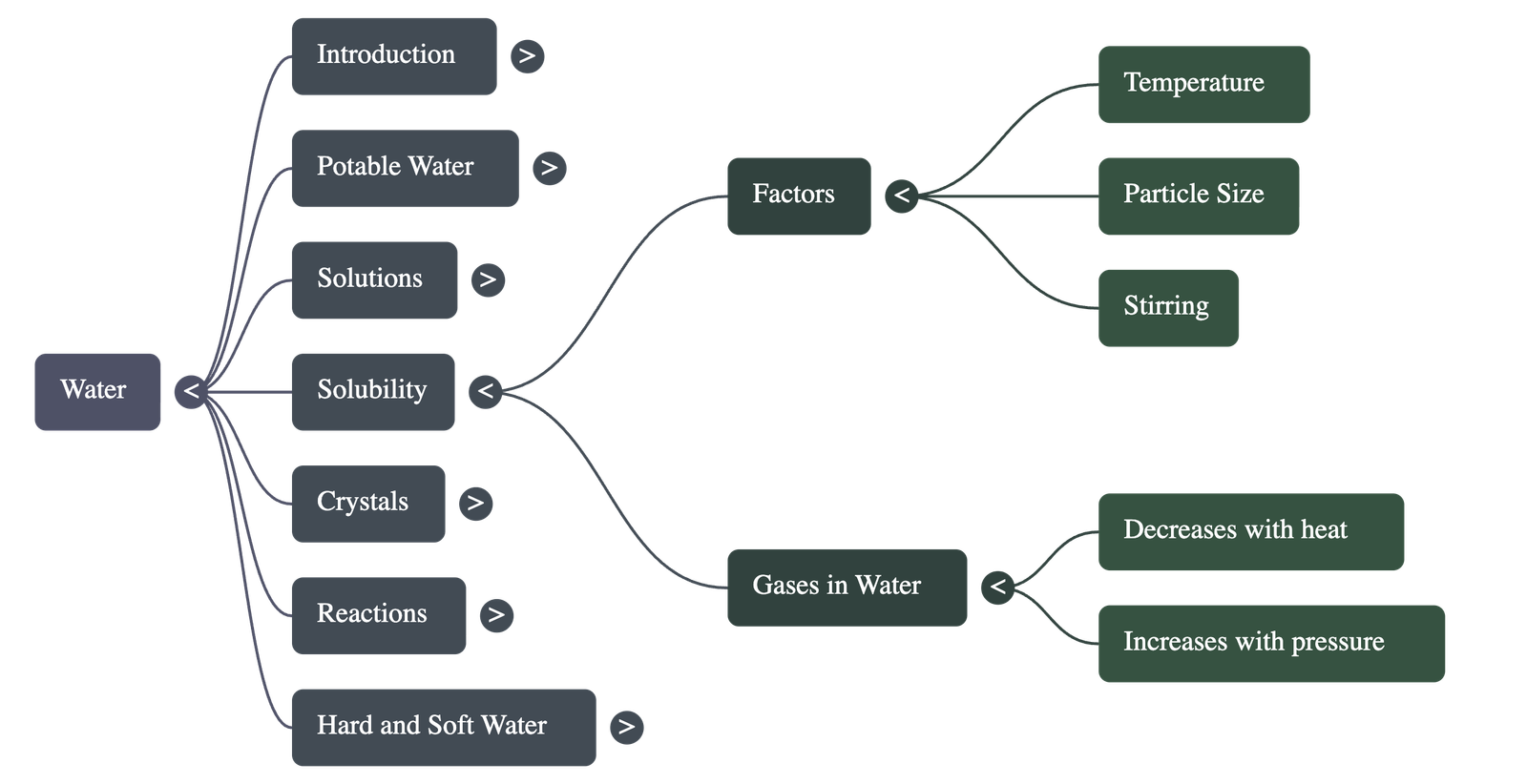
Solubility and Its Conditions
- Solubility is defined as the maximum amount of solute (in grams) that can saturate 100g of water at a specific temperature.
- Factors affecting solubility include particle size (powdered solids dissolve faster), stirring, and temperature.
- For most solids, solubility increases with temperature, whereas for gases, solubility is inversely proportional to temperature (boiling water removes dissolved gases, making it taste "flat").
- Solubility of gases is directly proportional to pressure, which is why soda fizzes when the bottle is opened and pressure is released.
Classification of Solutions
- True Solutions: Homogeneous mixtures where particles are < 1 nm and can pass through filter paper and membranes.
- Colloidal Solutions: Heterogeneous mixtures with particles between 1–100 nm; they show the Tyndall effect (scattering of light) and Brownian movement (random particle motion).
- Suspensions: Heterogeneous mixtures with particles > 100 nm that settle down over time and cannot pass through filter paper.
Saturation Levels
- Unsaturated Solution: A solution that can dissolve more solute at a given temperature.
- Saturated Solution: A solution that cannot dissolve any more solute at that specific temperature.
- Supersaturated Solution: A solution that contains more solute than it would normally hold at that temperature, often prepared by cooling a hot saturated solution.
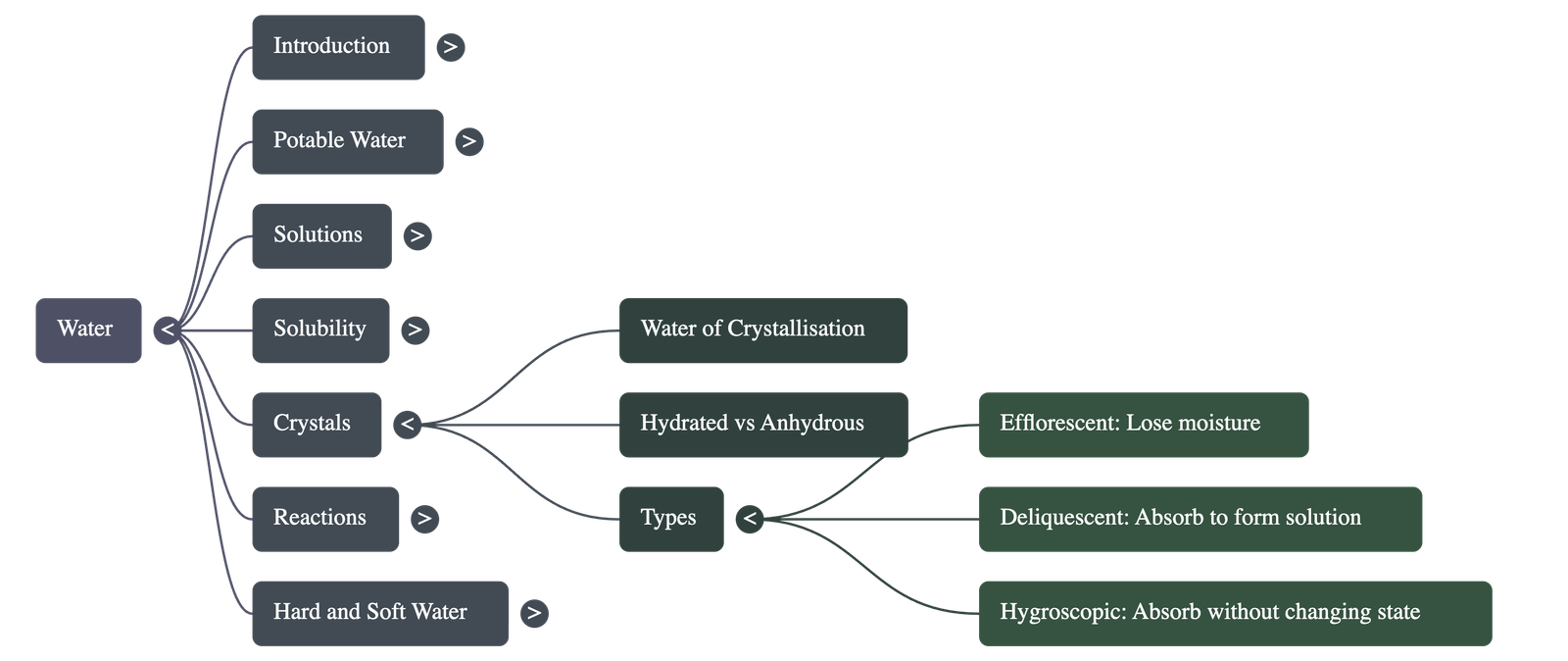
Crystals and Hydration
- Crystals are solids with definite regular shapes and symmetrical arrangements.
- Water of Crystallisation is the fixed number of water molecules that chemically combine with a salt to give it its shape and color.
- Hydrated crystals (like Blue Vitriol) contain this water, while Anhydrous crystals (like Potassium Nitrate) do not.
- Efflorescent substances lose moisture to the atmosphere (e.g., washing soda), Deliquescent substances absorb moisture and dissolve in it (e.g., caustic soda), and Hygroscopic substances absorb moisture without changing state (e.g., quicklime).
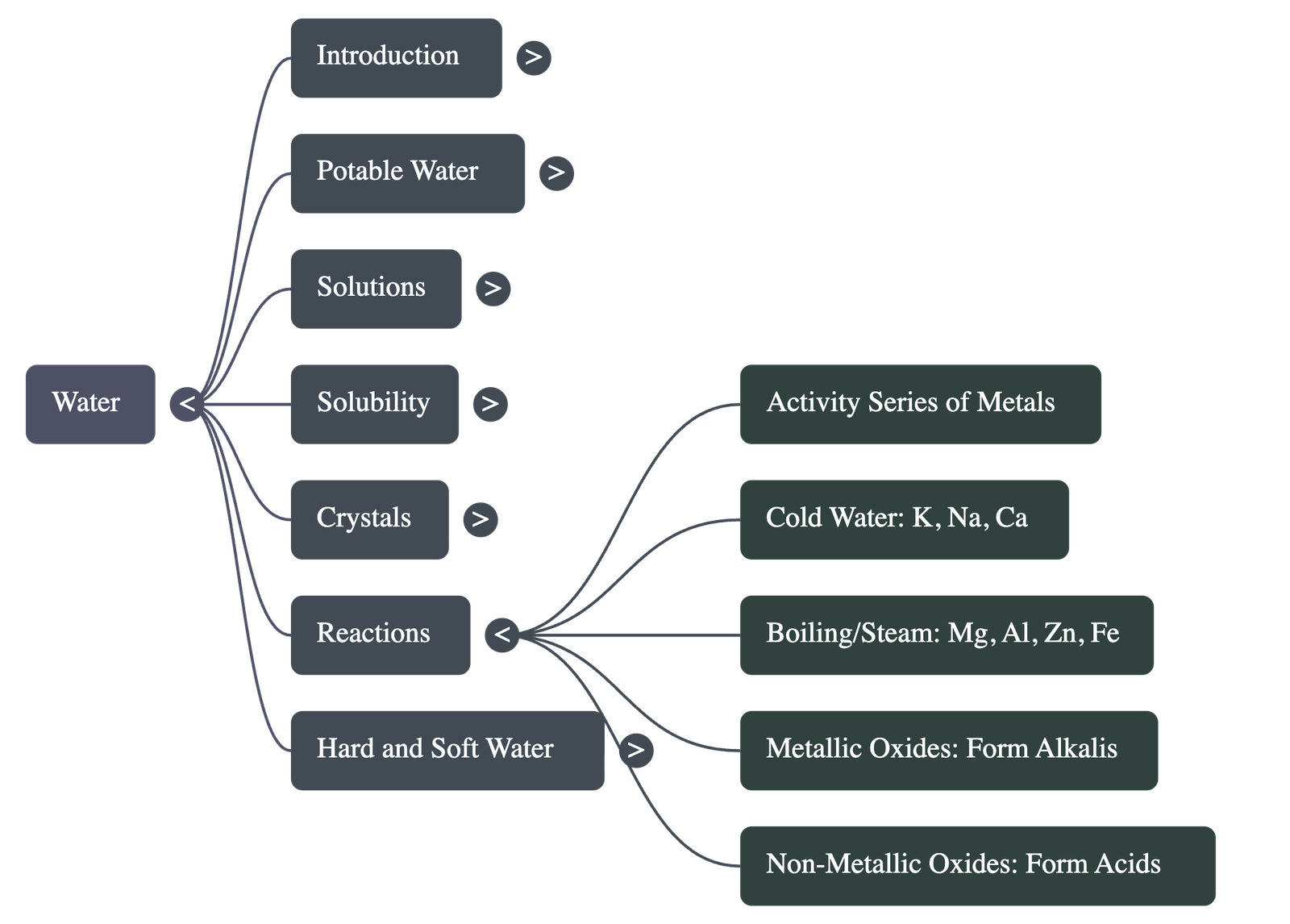
Chemical Reactions of Water
- Metals react based on the Activity Series: Potassium, Sodium, and Calcium react vigorously with cold water to release hydrogen gas.
- Magnesium, Aluminium, Zinc, and Iron react with boiling water or steam to form oxides and hydrogen.
- Metals below hydrogen (Copper, Mercury, Gold) do not react with water or steam.
- Metallic oxides react with water to form alkalis (bases), while non-metallic oxides react to form acids.
Hard and Soft Water
- Hard water does not lather easily with soap because it contains dissolved calcium and magnesium salts.
- Temporary Hardness is caused by bicarbonates of calcium and magnesium and can be removed by boiling.
- Permanent Hardness is caused by chlorides and sulphates of calcium and magnesium and cannot be removed by boiling; it requires the addition of washing soda.
- Hard water is disadvantageous as it wastes soap and forms "scale" or "fur" in industrial boilers.
Quick Navigation:
| | | | |
1 / 1
Quick Navigation:
| | | | |
Quick Navigation:
| | | | |