Quick Navigation:
| | | | |
Quick Navigation:
| | | | |
Atomic Structure
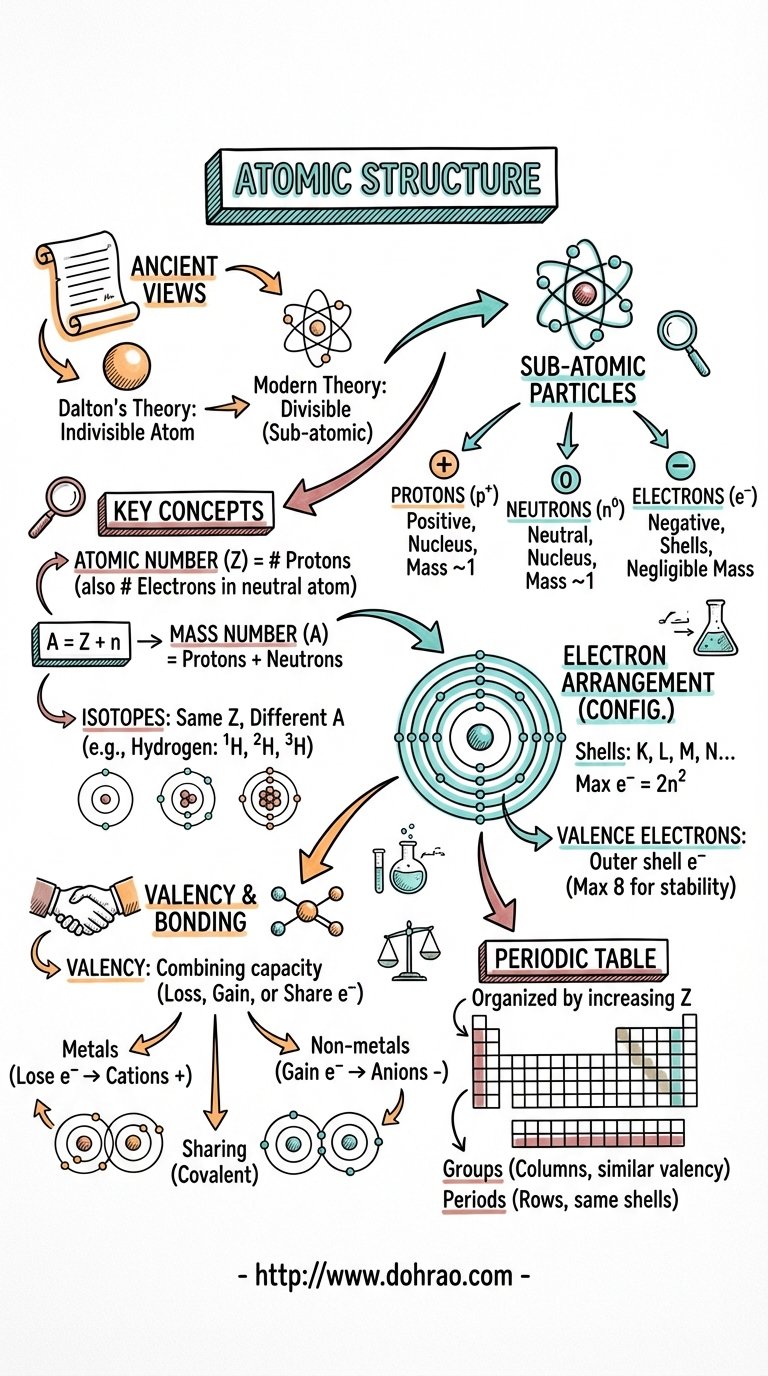
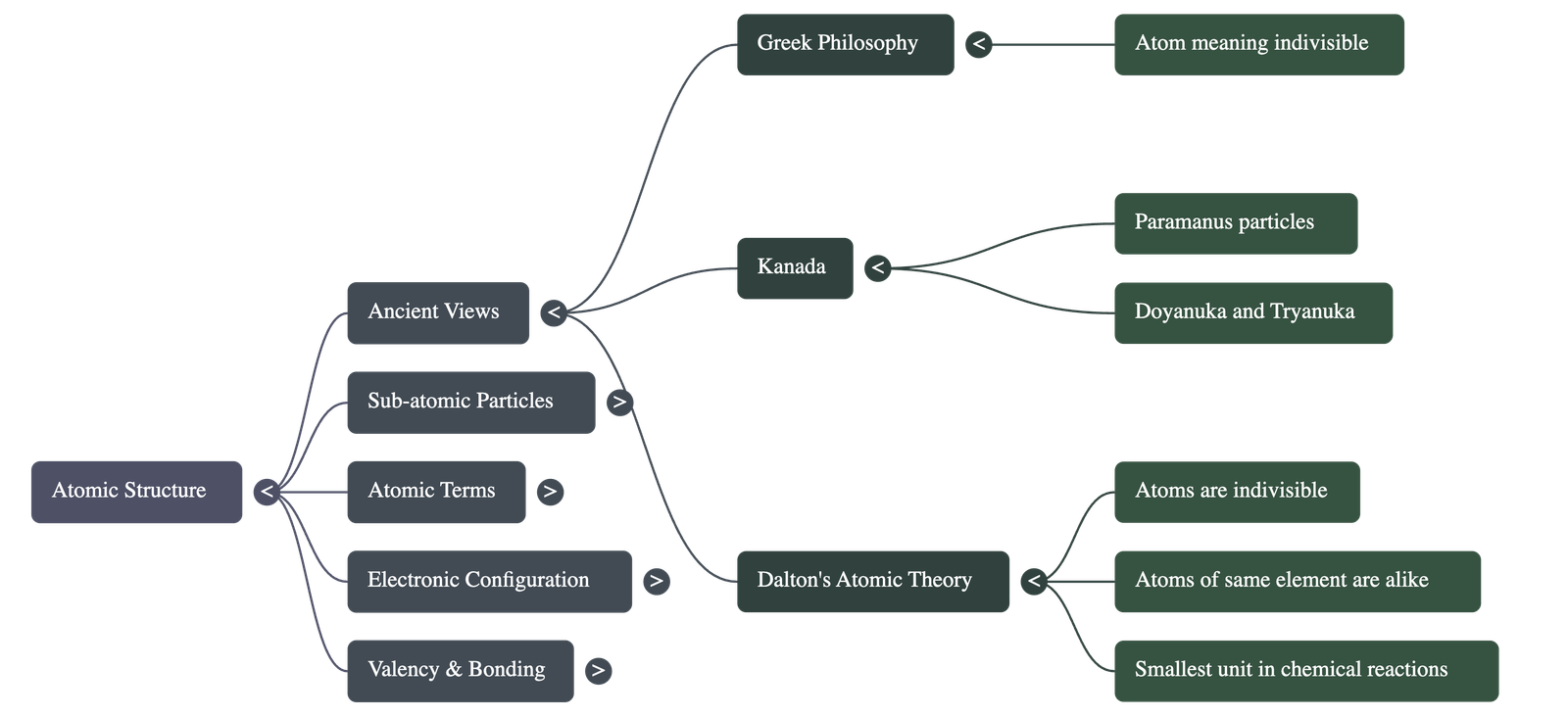
1. Ancient and Historical Views
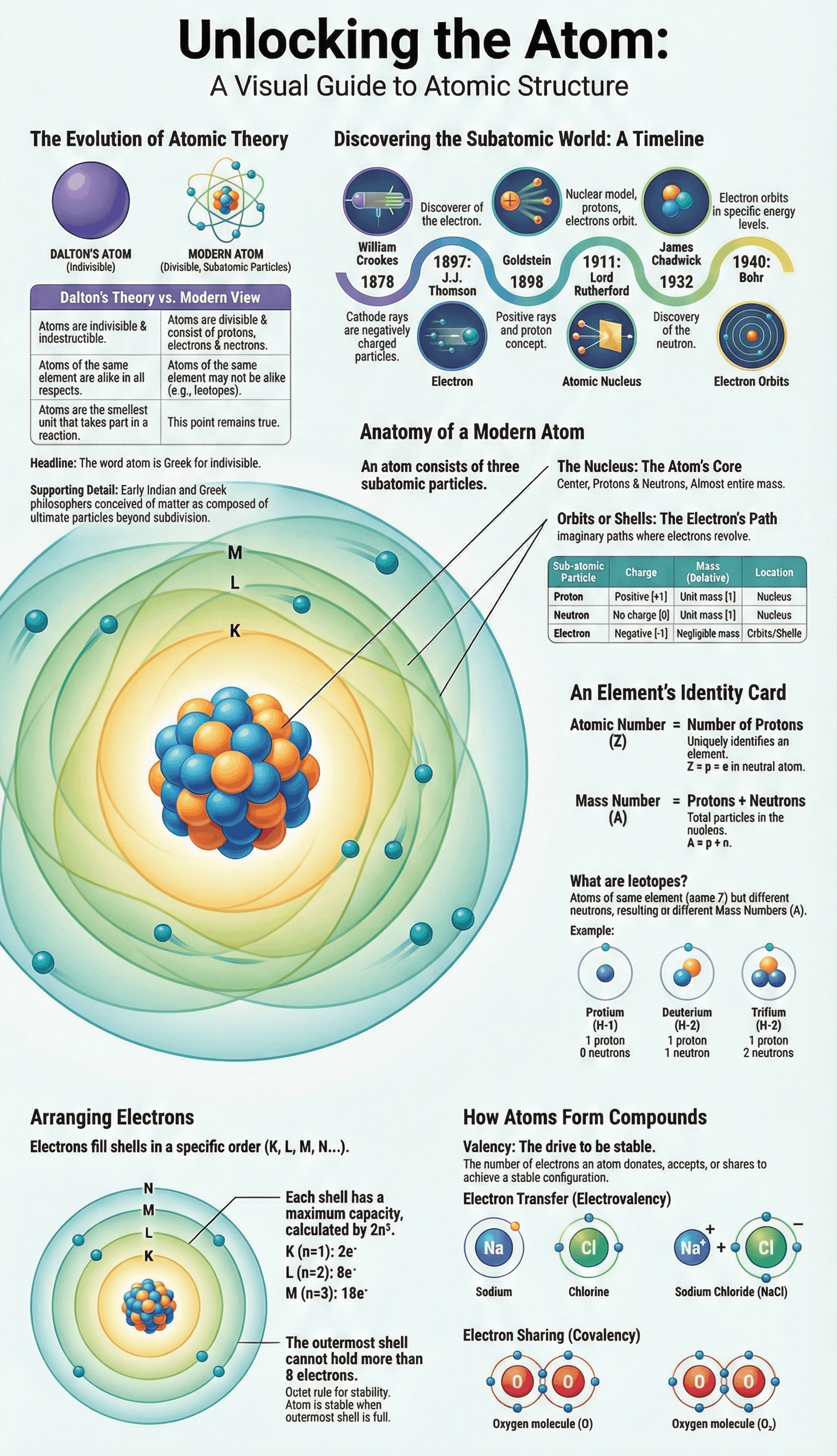
- Origin of 'Atom': Derived from the Greek word meaning 'indivisible', signifying a particle that cannot be further subdivided.
- Early Philosophers: The concept of matter being made of tiny particles was first proposed by Indian philosopher Kanada (who called them paramanus) and Greek philosophers.
- Dalton's Atomic Theory: John Dalton postulated that atoms are indivisible, indestructible, and that atoms of the same element are identical.
- Modern Atomic Theory: Contradicts Dalton by proving atoms are divisible (consisting of subatomic particles), destructible, and that atoms of the same element can differ (isotopes).
2. Subatomic Particles & Their Discovery
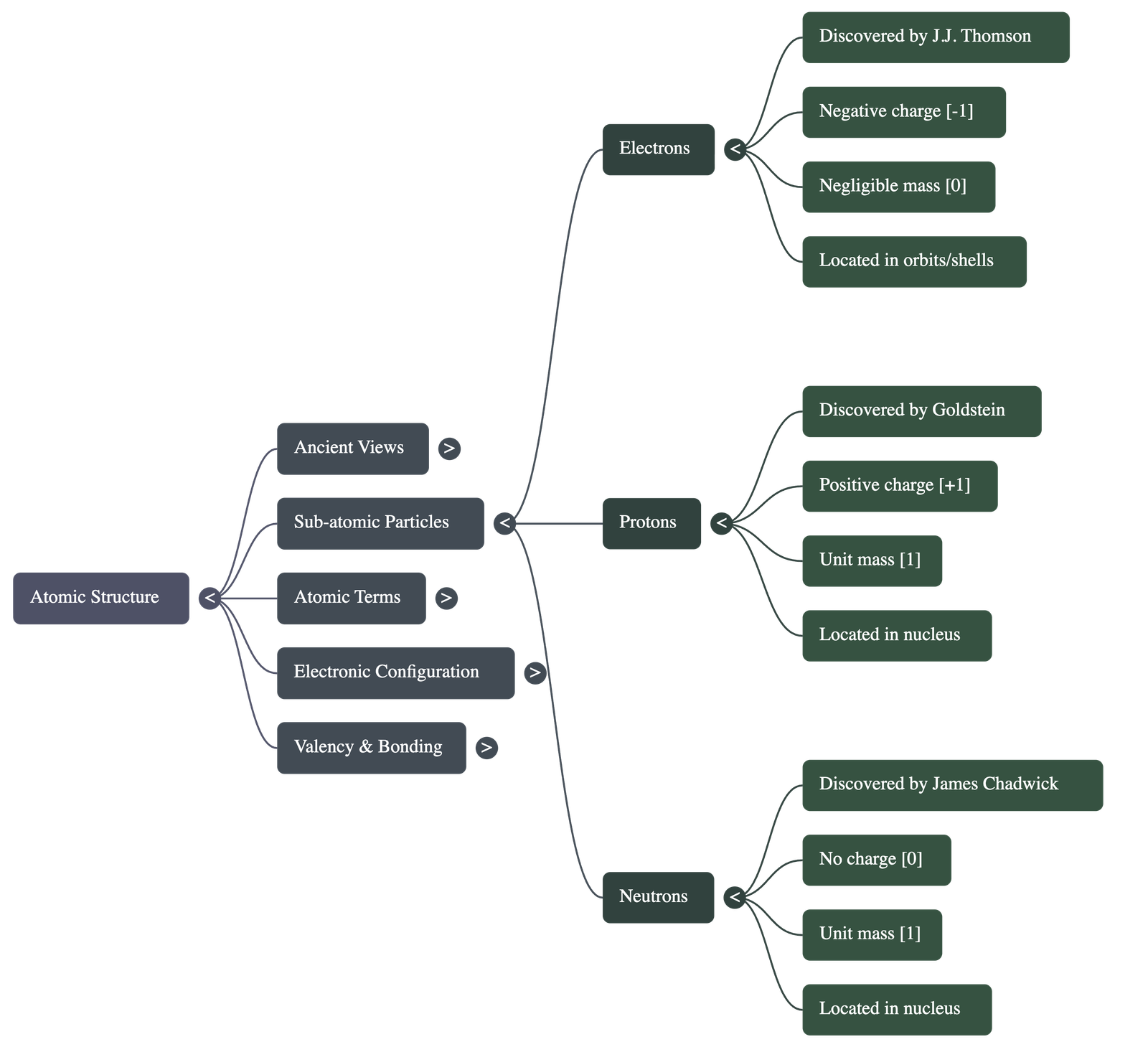
- Electrons: Discovered by William Crookes and J.J. Thomson through cathode ray experiments. They are negatively charged and have negligible mass.
- Protons: Discovered by Goldstein using modified cathode ray tubes (anode rays). They are positively charged and have unit mass.
- Atomic Nucleus: Discovered by Lord Rutherford via the alpha particle scattering experiment, proving that the centre of an atom is a concentrated positive mass.
- Neutrons: Discovered by James Chadwick. These are neutral particles found in the nucleus with a mass nearly equal to that of a proton.
3. Key Atomic Terms
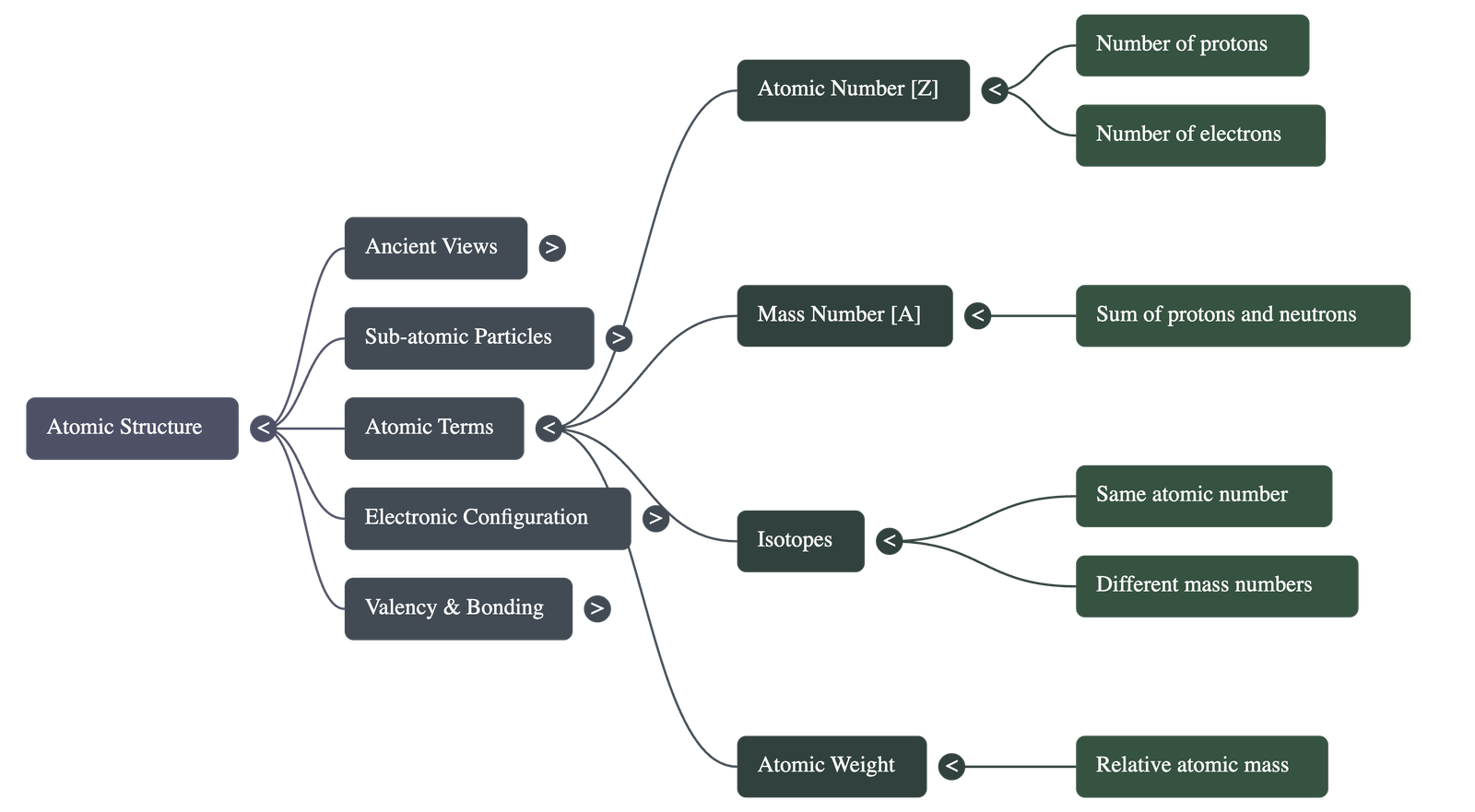
- Atomic Number (Z): The total number of protons in the nucleus of an atom. In a neutral atom, this also equals the number of electrons.
- Mass Number (A): The sum of the number of protons and neutrons in the nucleus.
- Isotopes: Atoms of the same element that have the same atomic number but different mass numbers (e.g., Protium, Deuterium, and Tritium for Hydrogen).
- Atomic Weight: A relative measure of how many times an atom of an element is heavier than 1/12th of the mass of a carbon atom.
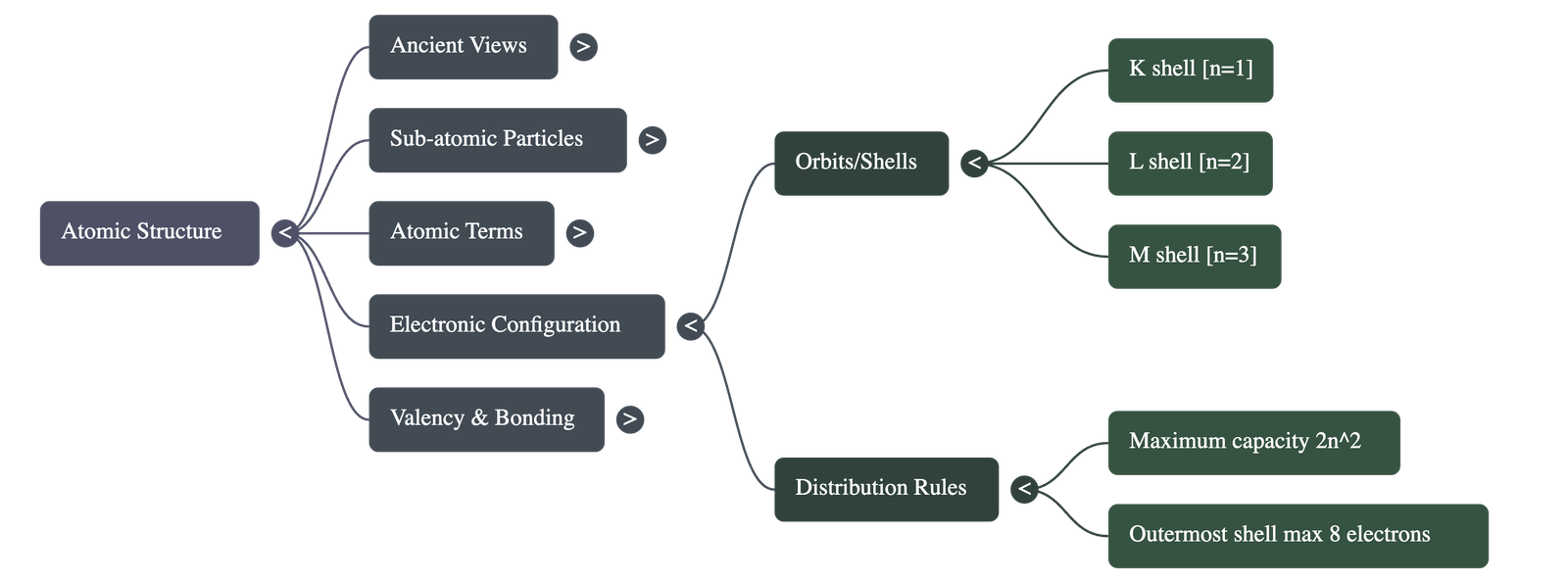
4. Arrangement of Electrons
- Orbits/Shells: Electrons revolve around the nucleus in fixed imaginary paths called orbits or energy levels, labelled as K, L, M, N...
- 2n² Rule: The maximum number of electrons in a shell is determined by the formula 2n², where 'n' is the shell number.
- Octet Rule: The outermost shell of an atom cannot accommodate more than 8 electrons.
- Electronic Configuration: The systematic distribution of electrons in different shells (e.g., Sodium with 11 electrons is arranged as 2, 8, 1).
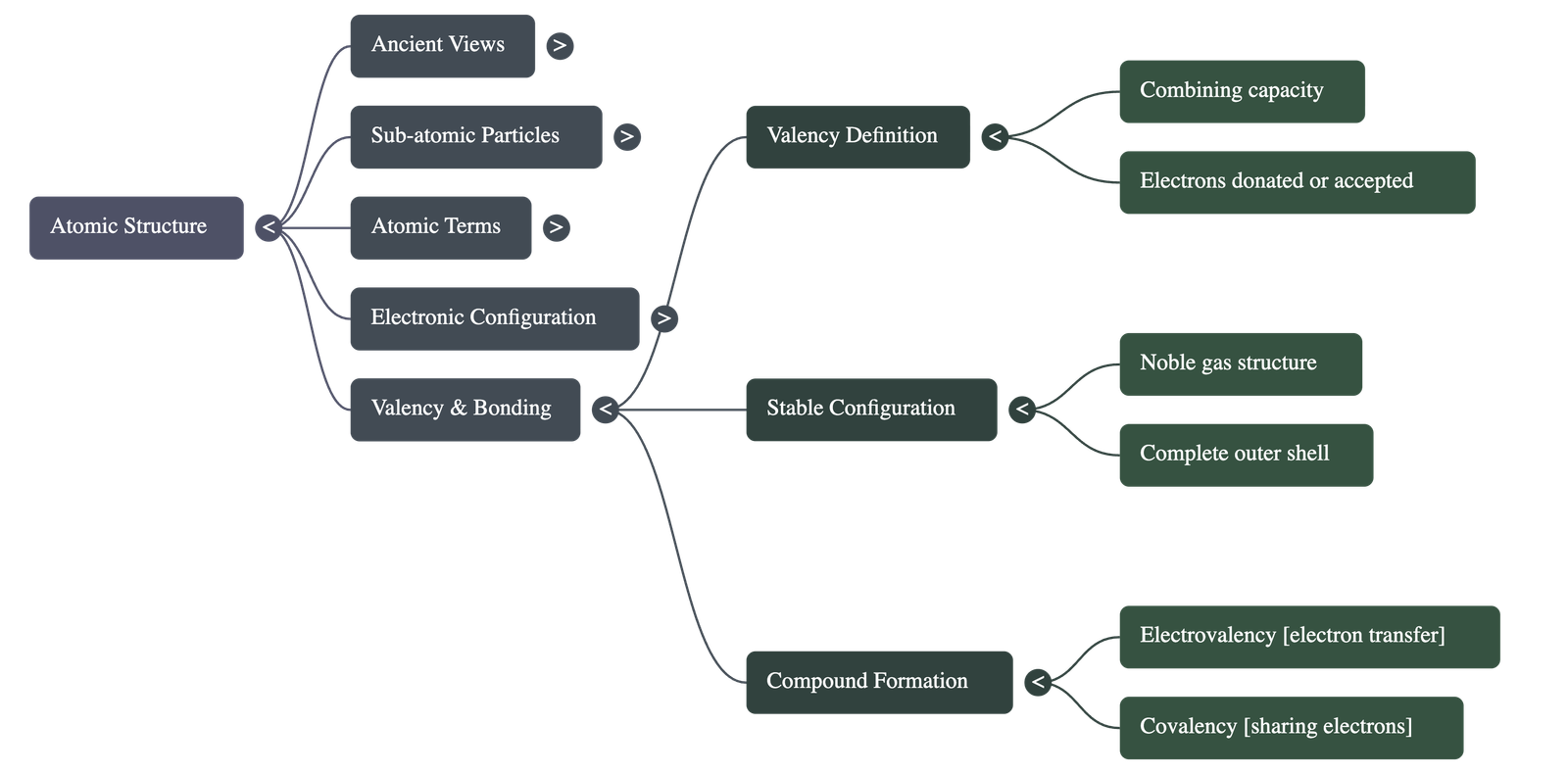
5. Valency and Chemical Bonding
- Definition: Valency is the combining capacity of an atom, determined by the number of electrons it loses, gains, or shares to achieve stability.
- Stable Configuration: Noble gases (like Helium, Neon, Argon) are stable because they have a complete outer shell (2 or 8 electrons).
- Metals (Cations): Elements with 1, 2, or 3 electrons in their outer shell tend to lose electrons to become positively charged ions.
- Non-metals (Anions): Elements with 5, 6, or 7 electrons in their outer shell tend to gain electrons to become negatively charged ions.
- Compound Formation: Stability is achieved through Electrovalency (transfer of electrons) or Covalency (sharing of electrons).
Quick Navigation:
| | | | |
1 / 1
Quick Navigation:
| | | | |