Quick Navigation:
| | | | |
Quick Navigation:
| | | | |
Matter
1. Introduction and Definition
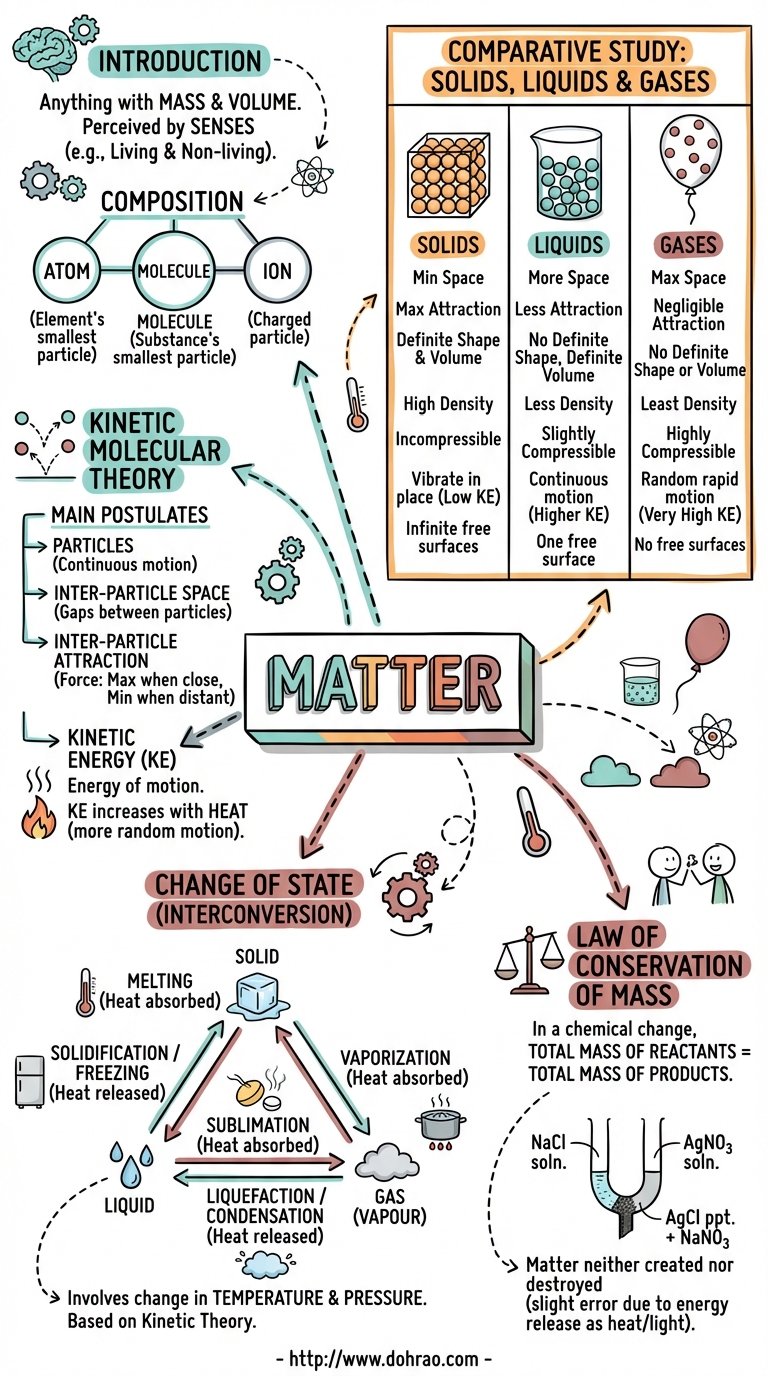
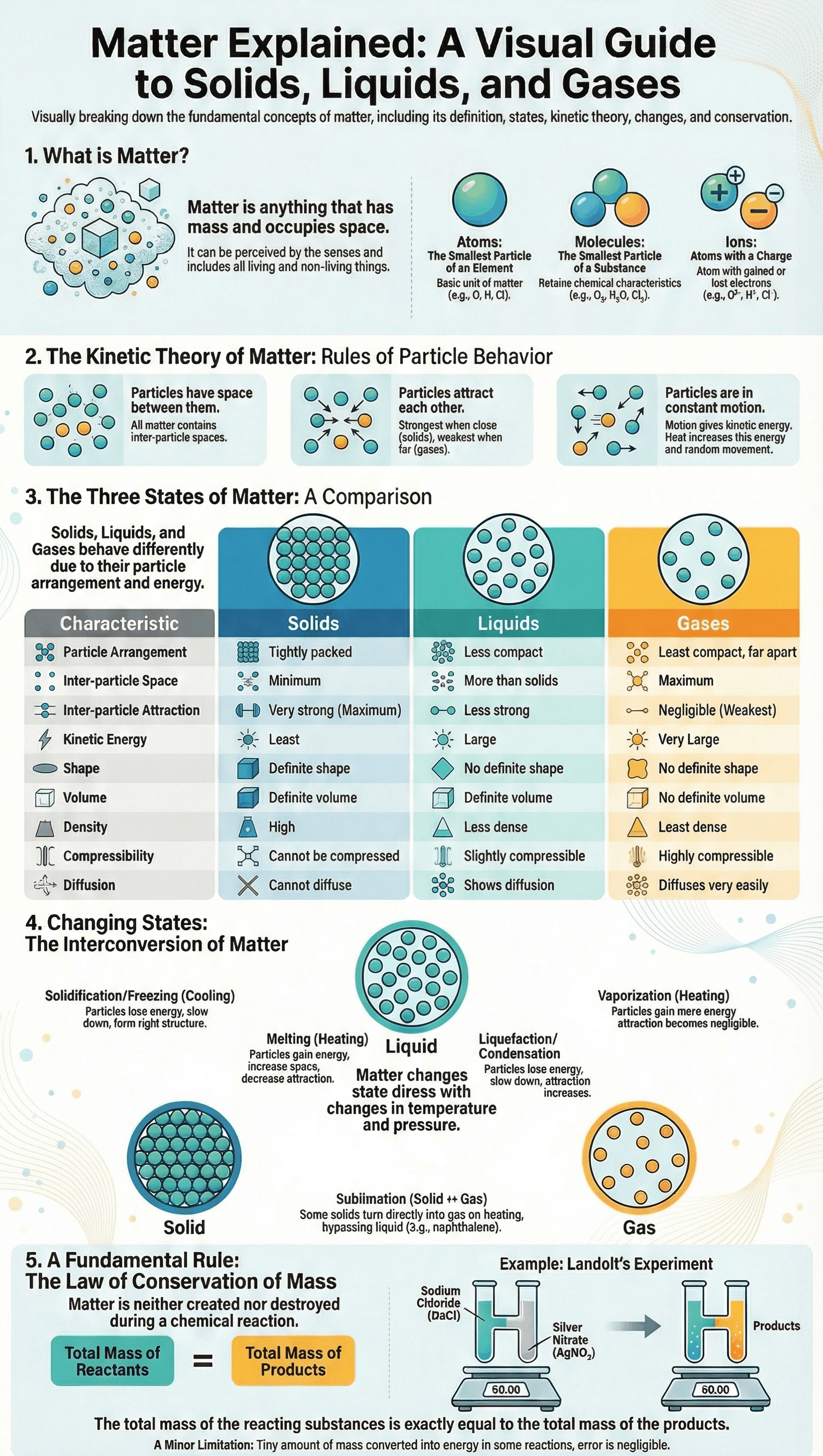
- Matter is anything that occupies space, has mass, and can be perceived by the senses.
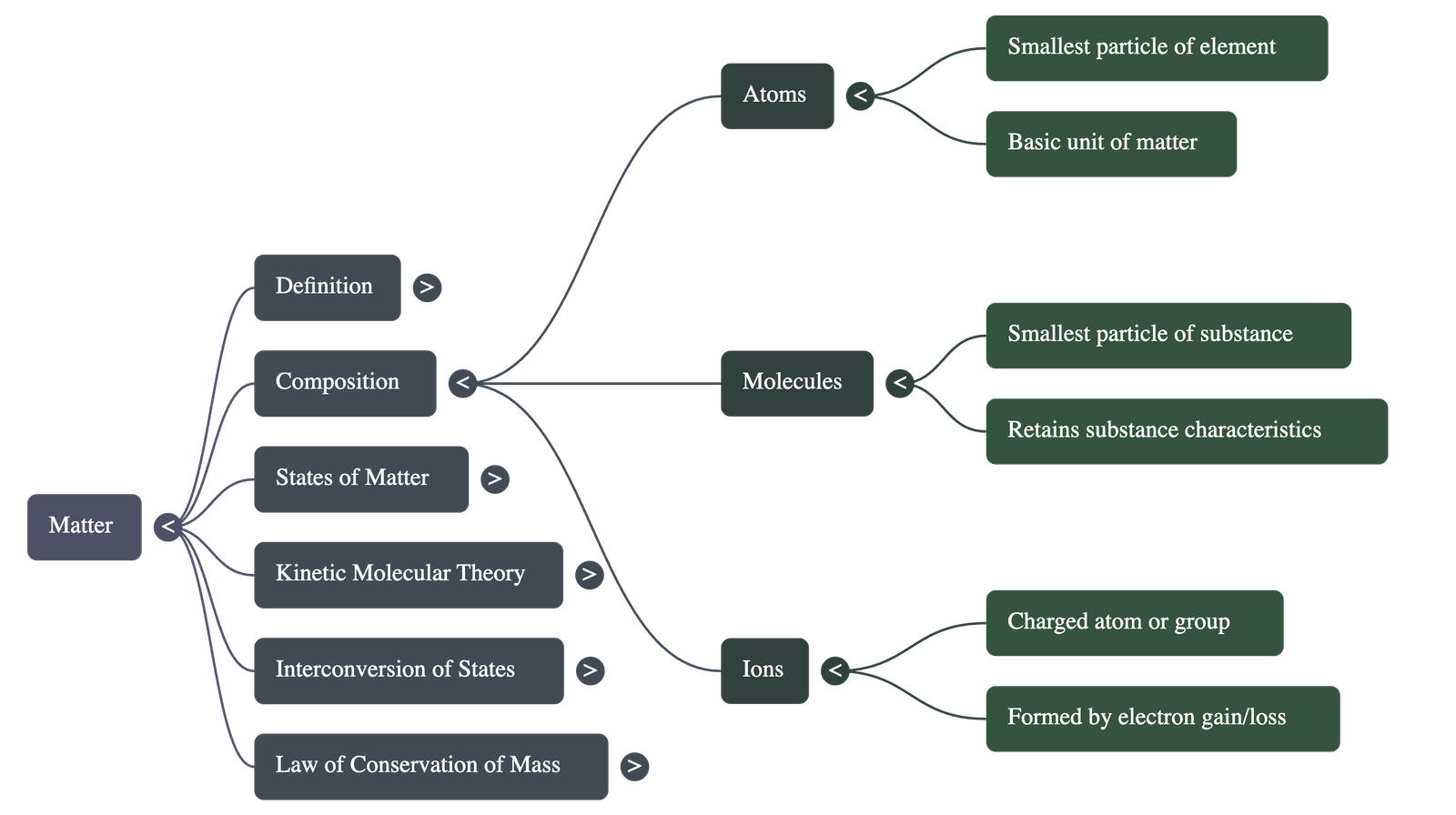
- It is composed of three types of small particles: atoms (smallest unit of an element), molecules (smallest unit of a substance retaining its characteristics), and ions (atoms or groups of atoms with a resultant charge).
- Key characteristics include volume (space occupied), mass (quantity of matter), and weight (gravitational pull on the matter).
2. States of Matter
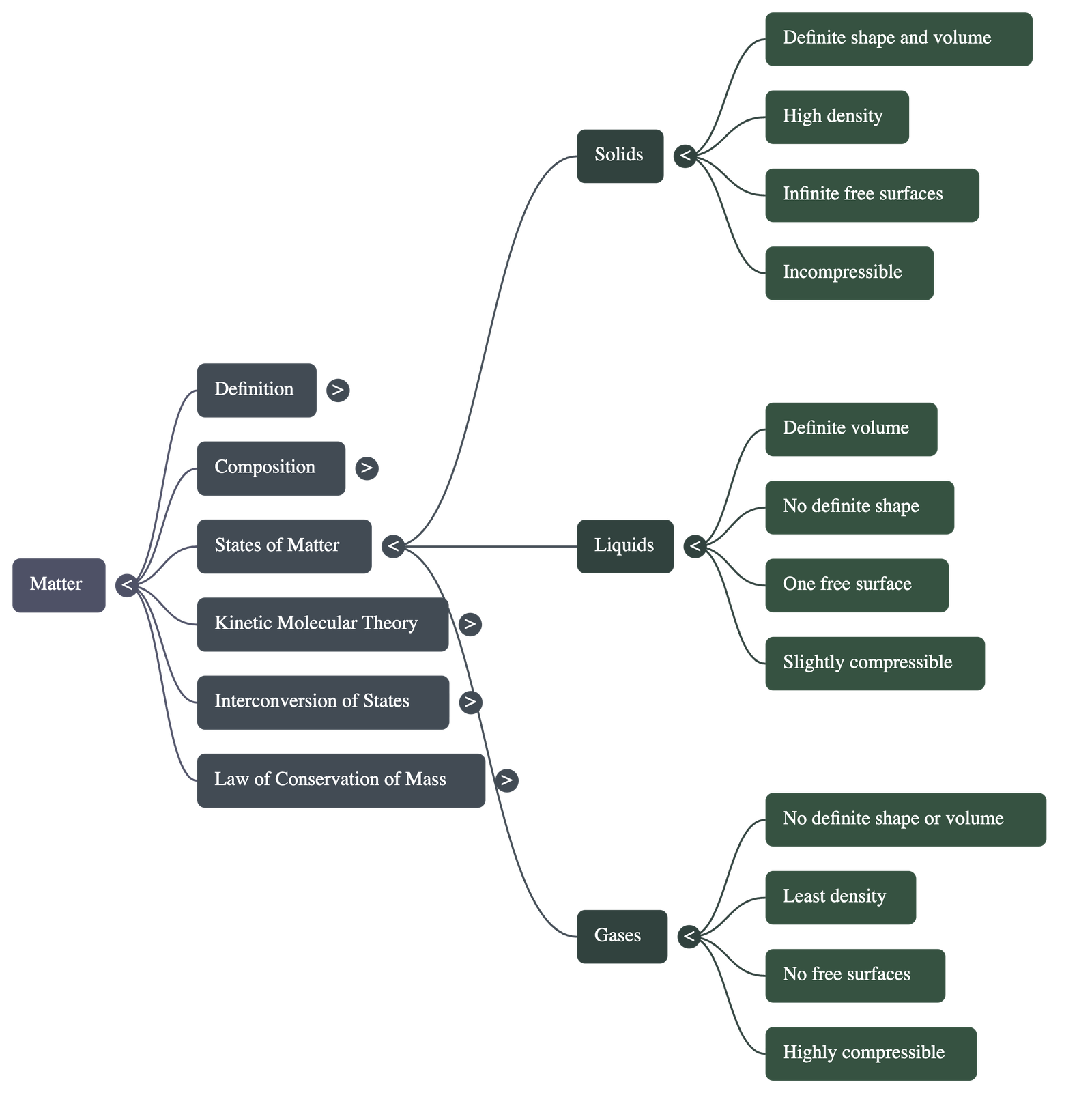
- Solids: Have a definite volume and shape, high density, and infinite free surfaces. They cannot be compressed or diffused.
- Liquids: Have a definite volume but no definite shape (taking the shape of their container). they have one upper free surface, are slightly compressible, and show diffusion.
- Gases: Have no definite volume or shape. They have the least density, no free surfaces, and are highly compressible. Gases diffuse very easily and rapidly.
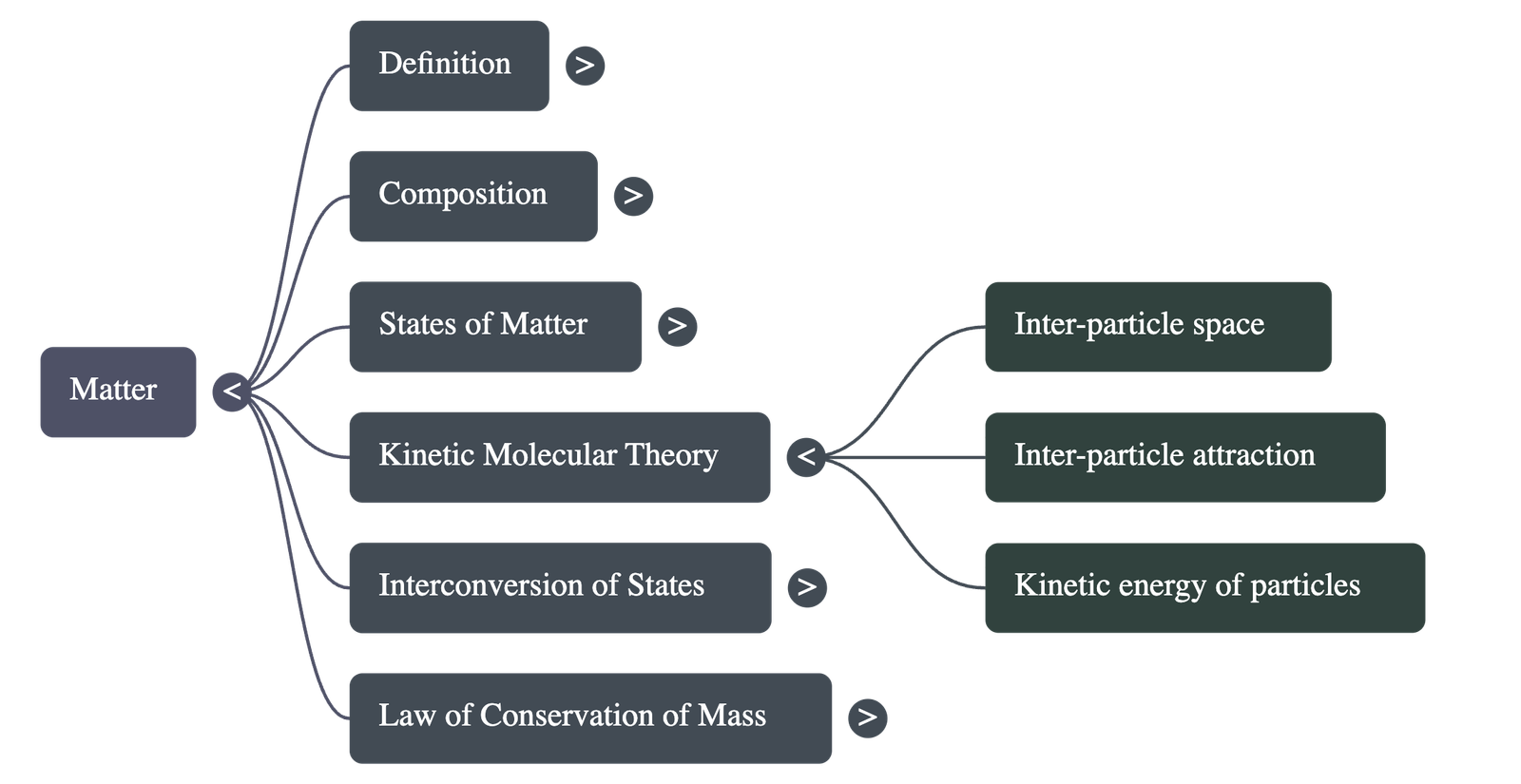
3. Kinetic Molecular Theory of Matter
- Postulates: Matter is made of particles in continuous motion, possessing kinetic energy. These particles have spaces between them (inter-particle space) and attract each other (inter-particle attraction).
- Solids: Particles are closely packed with minimum space, maximum attraction, and least kinetic energy.
- Liquids: Particles are less compact with more space than solids, less attraction, and large kinetic energy.
- Gases: Particles are least compact with maximum space, negligible attraction, and very large kinetic energy.
4. Interconversion of State
- Melting: Solid changes to liquid on heating as particles gain energy and overcome attraction.
- Vaporisation: Liquid changes to gas on heating; particles gain energy until attraction becomes negligible.
- Liquefaction (Condensation): Gas changes to liquid on cooling as particles lose energy and move closer.
- Solidification (Freezing): Liquid changes to solid on cooling; particles lose energy and attraction increases.
- Sublimation: Solid changes directly into a gas (and vice versa) without passing through the liquid state, common in substances with very low inter-particle attraction like naphthalene or ammonium chloride.
5. Law of Conservation of Mass
- Principle: Formulated by Lavoisier, it states that matter is neither created nor destroyed during a chemical reaction; the total mass of reactants equals the total mass of products.
- Experimental Evidence: Landolt's experiment used an H-shaped tube to react sodium chloride and silver nitrate, proving the weight remained constant before and after the reaction.
- Limitations: In some cases, mass can be converted to energy (heat or light), so mass and energy should strictly be considered together.
Quick Navigation:
| | | | |
1 / 1
Quick Navigation:
| | | | |
Quick Navigation:
| | | | |