Quick Navigation:
| | | | |
Quick Navigation:
| | | | |
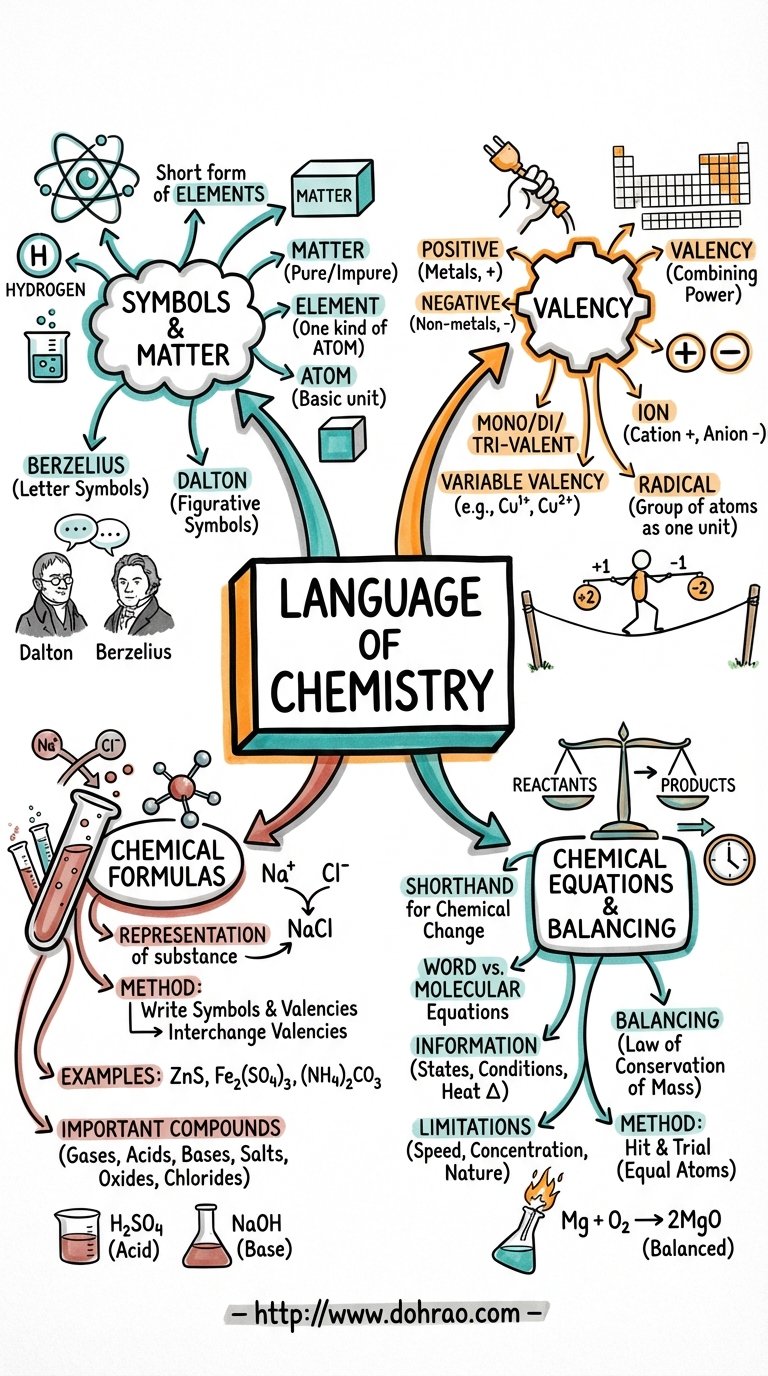
Language of Chemistry
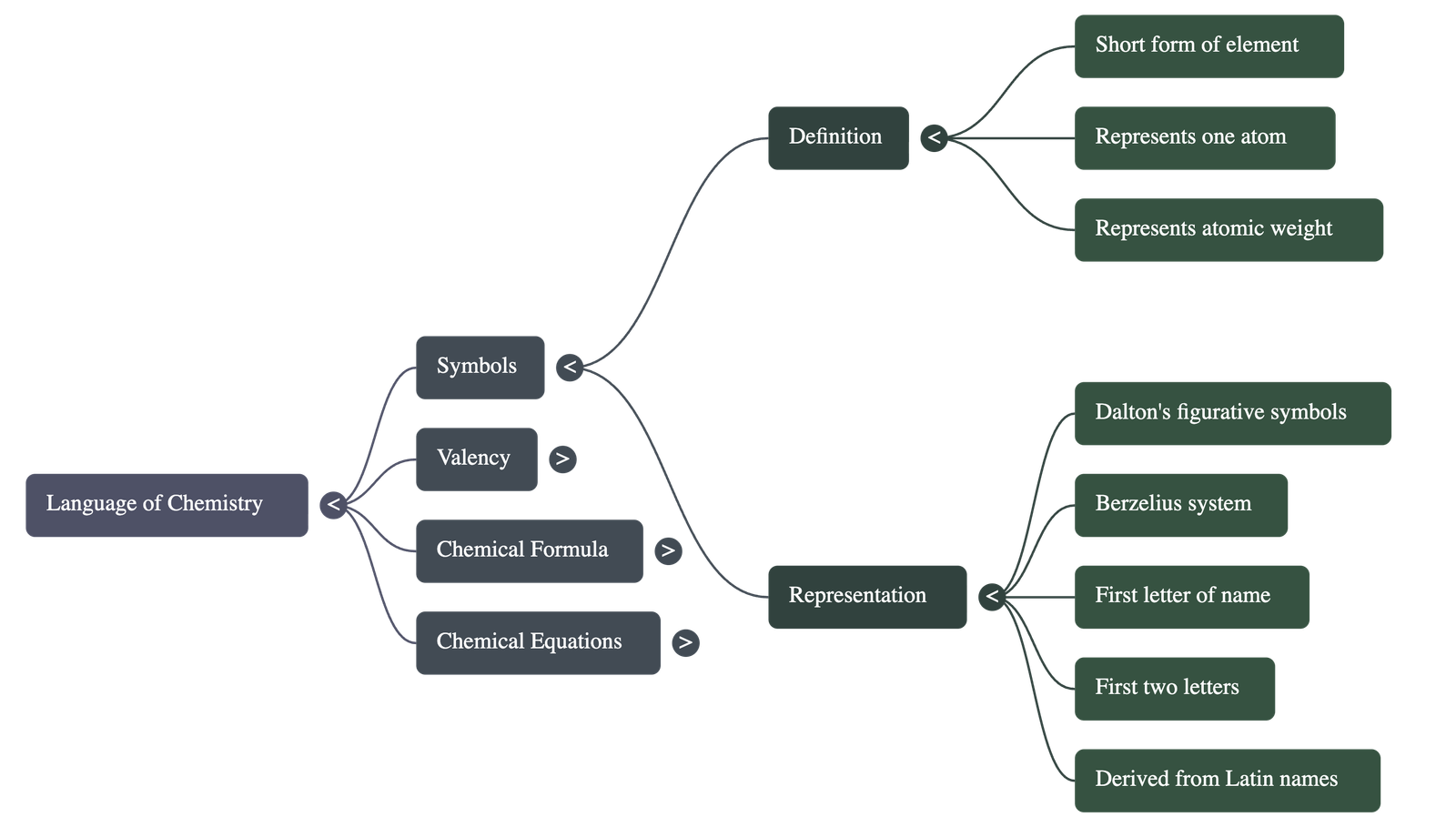
1. Representation of Elements: Symbols
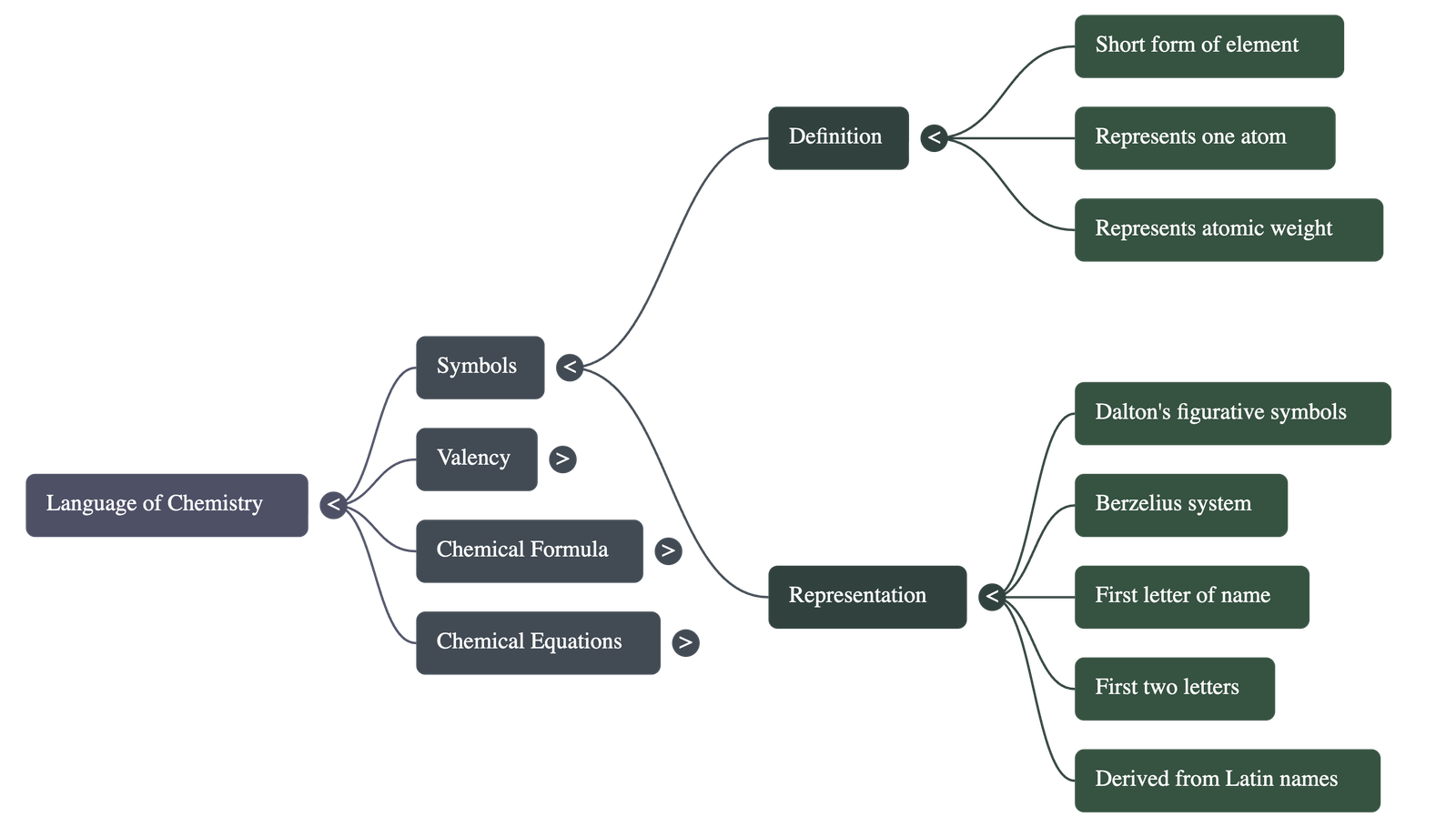
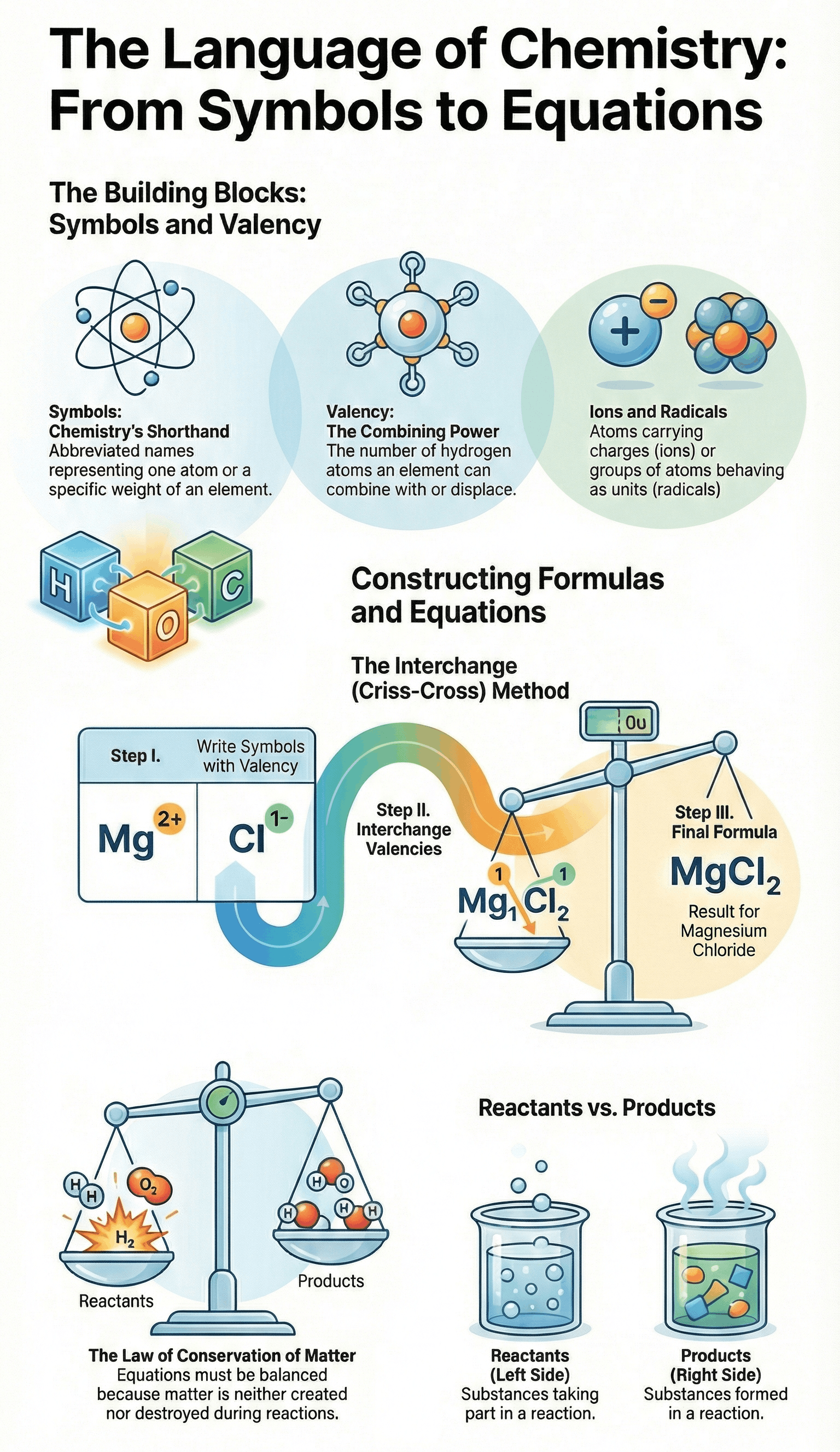
- A symbol is a short form or abbreviated name used to represent a specific element or one atom of that element.
- It signifies the atomic weight of the element and distinguishes one element from another.
- Historical Context: John Dalton suggested figurative symbols in 1807, while Jacob Berzelius suggested the modern approach in 1814.
- Naming Rules: Symbols are derived from the first letter of the element's name (e.g., C for Carbon), the first two letters (e.g., Ca for Calcium), or from its Latin name (e.g., Cu from Cuprum for Copper).
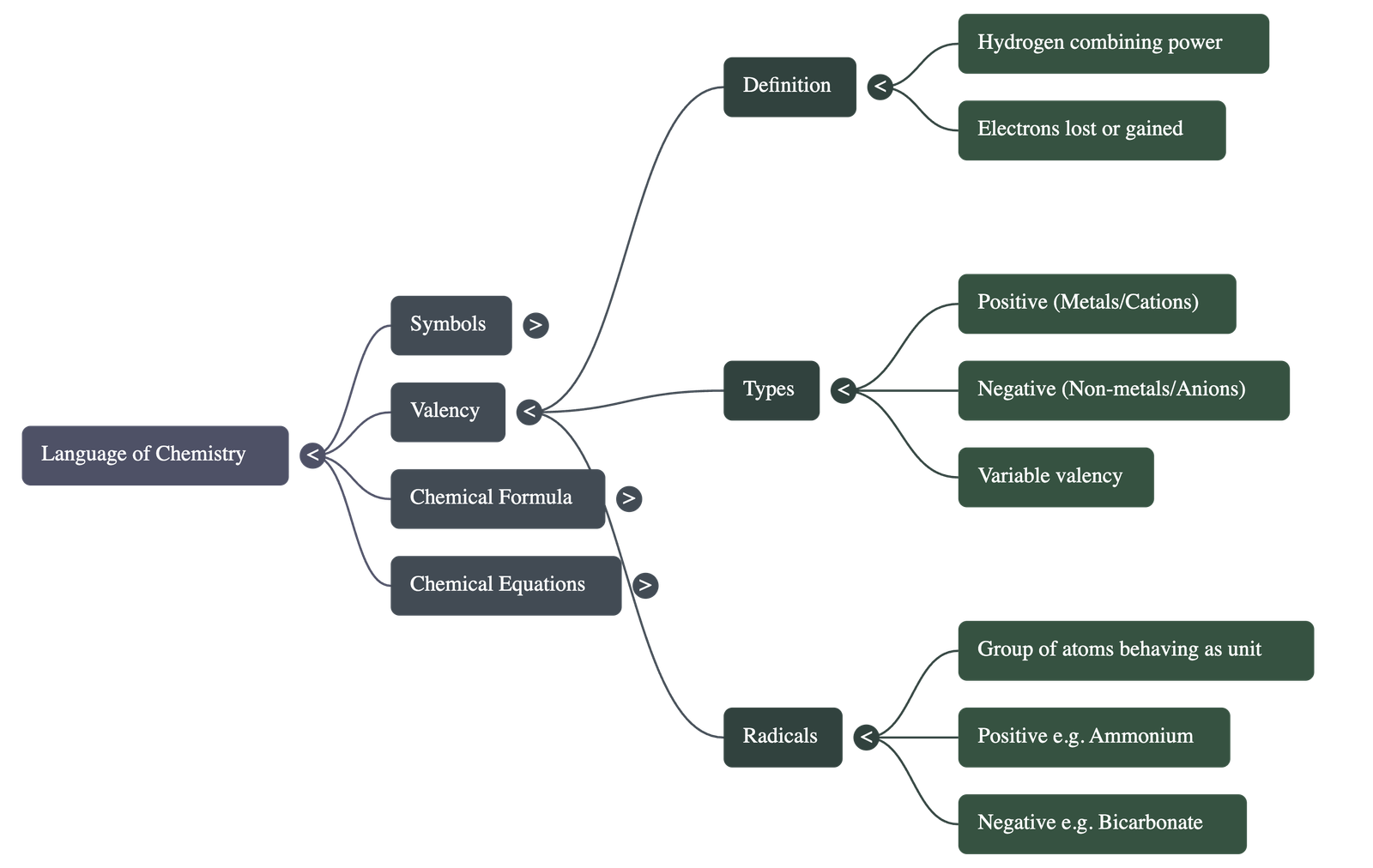
2. Valency and Combining Power
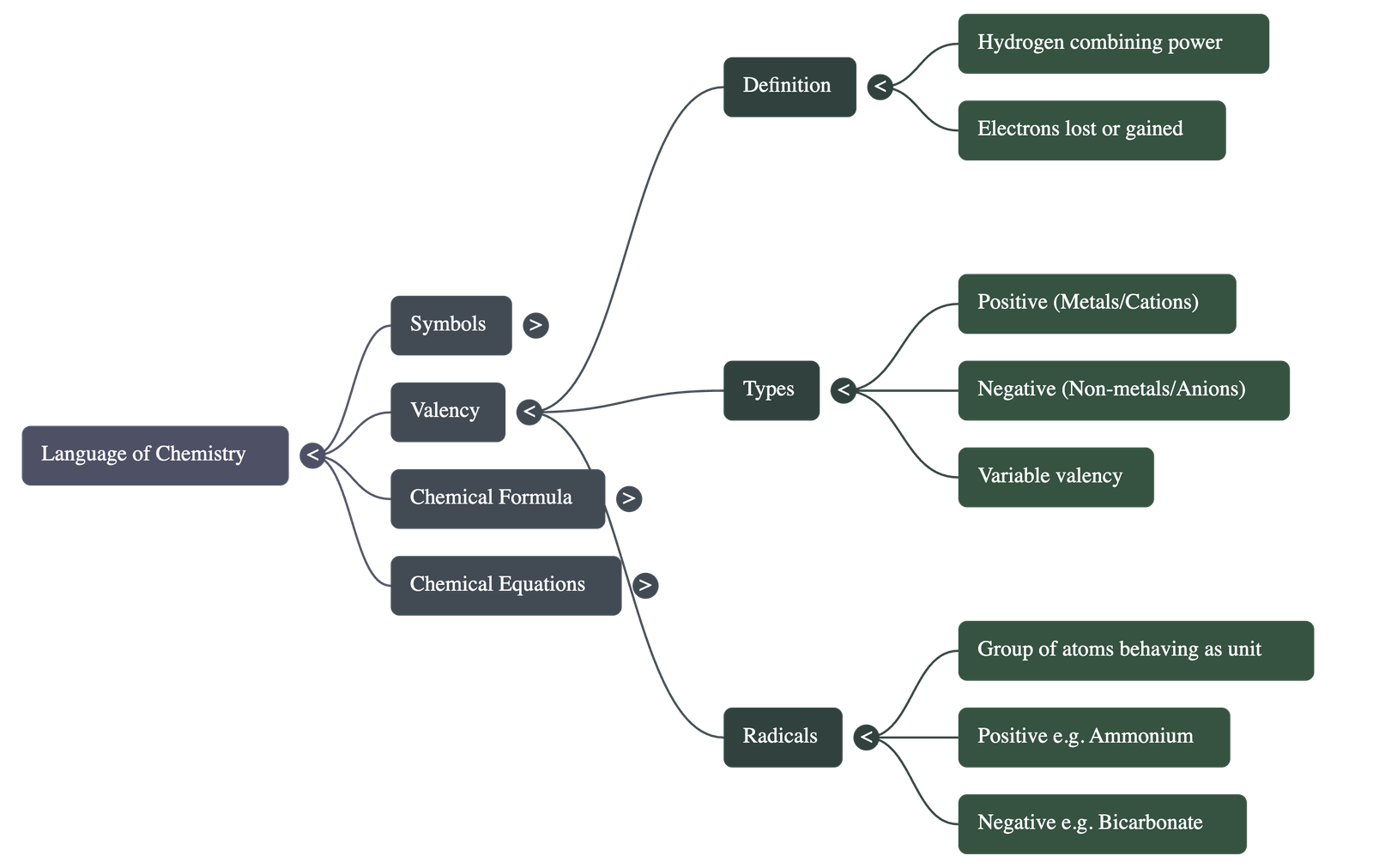
- Definition: Valency is the number of hydrogen atoms that can combine with or displace one atom of an element or radical to form a compound.
- Electronic Definition: It is the number of electrons an atom can donate or accept to achieve a stable electronic configuration similar to the nearest noble gas.
- Classification: Metals generally have positive valency (cations), while non-metals have negative valency (anions). Elements are described as monovalent, divalent, or trivalent based on their valency number.
- Variable Valency: Some elements exhibit more than one valency due to variations in the loss or gain of electrons from their outer shells (e.g., Iron can be Fe2+ or Fe3+).
3. Ions and Radicals
- An ion is an atom or group of atoms carrying a positive charge (cation) or a negative charge (anion) due to the loss or gain of electrons.
- A radical is a group of atoms of different elements that behave as a single unit and possess their own valency (e.g., Ammonium NH4+ or Sulphate SO42-).
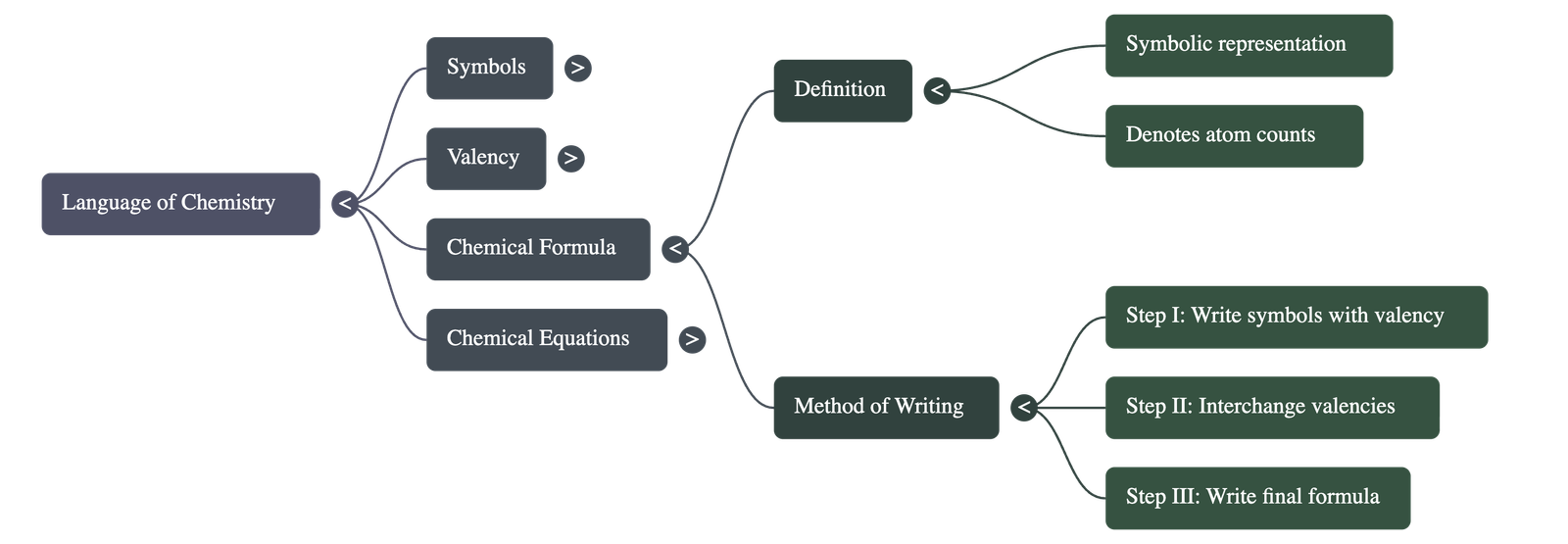
4. Chemical Formulae
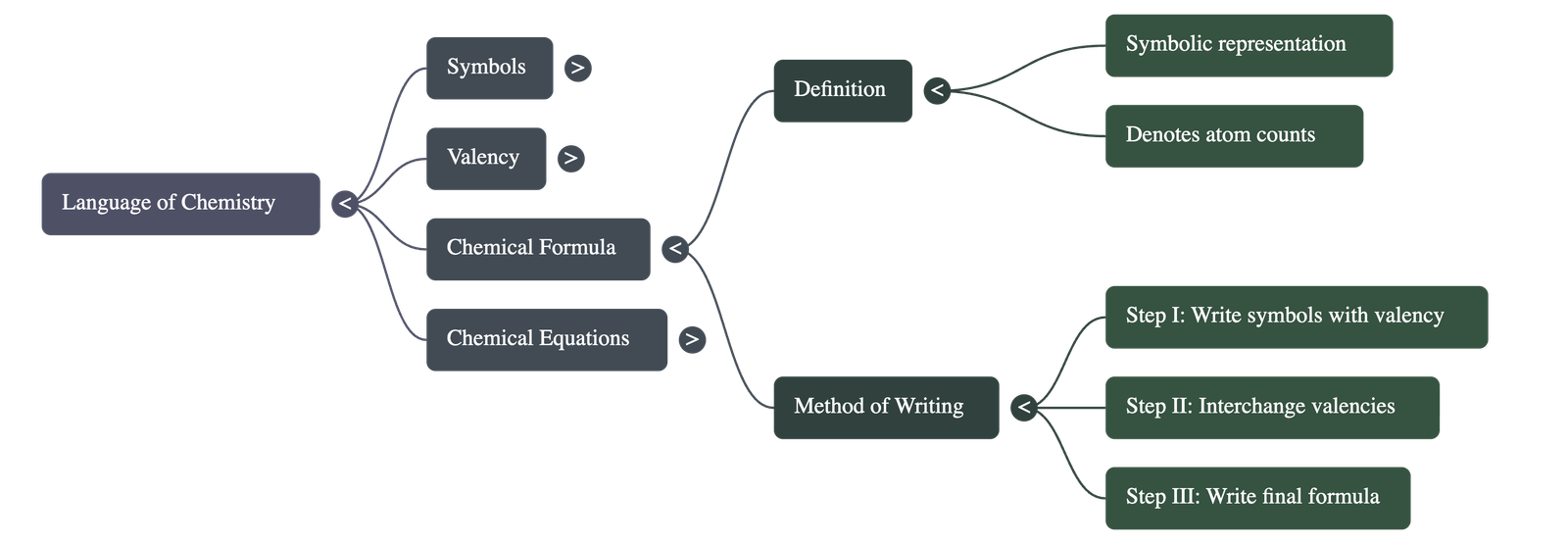
- A chemical formula represents a substance using symbols and denotes the number of atoms of each element present in a compound.
- Method of Writing: To write a formula, the symbols of the elements/radicals are written with their valencies. These valencies are then interchanged (criss-crossed) to determine the subscript numbers for each symbol.
- Positive ions are always written first in the formula.
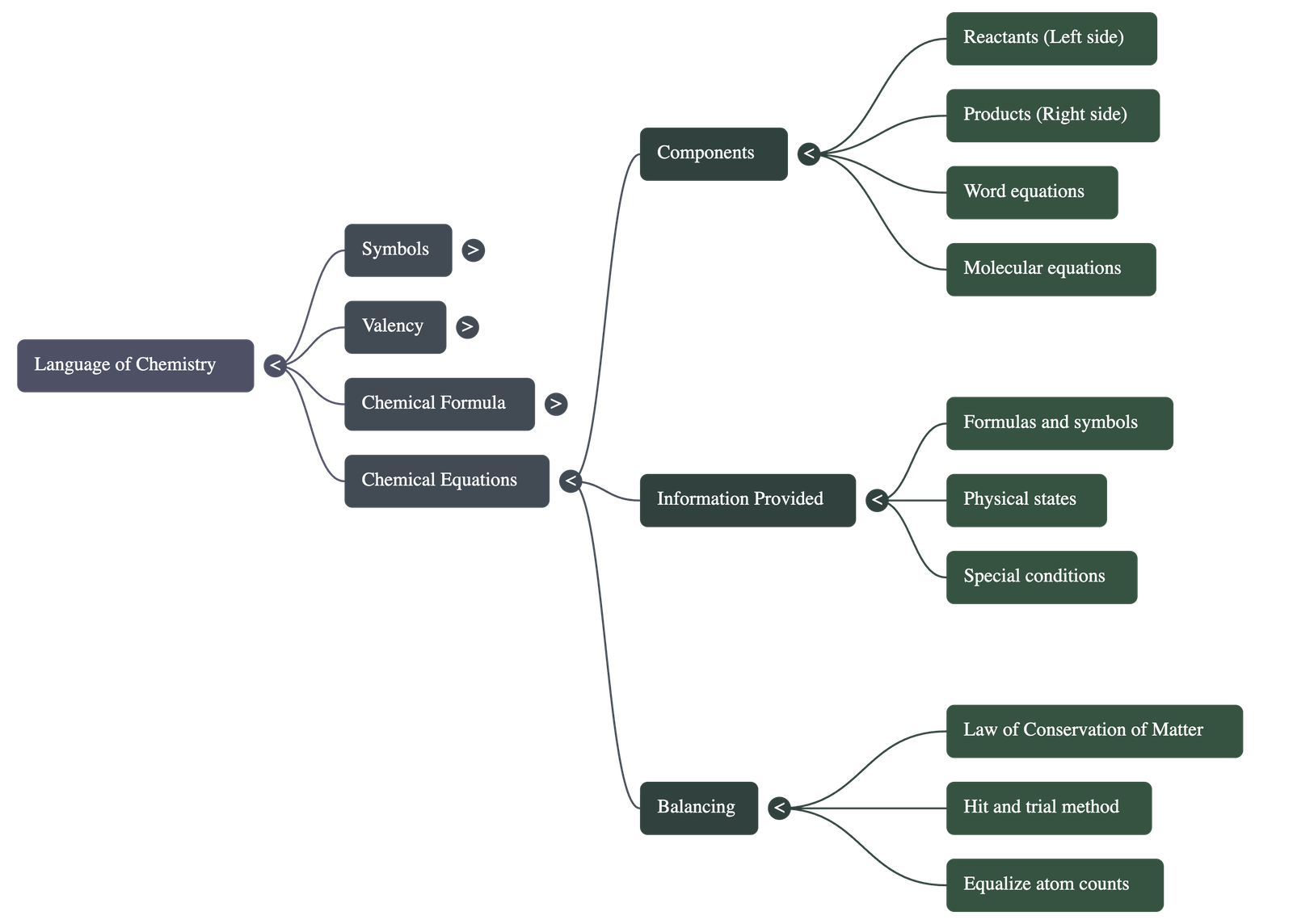
5. Chemical Equations
- A chemical equation is a shorthand representation of a chemical change using symbols and formulae.
- Reactants: Substances that take part in the reaction, written on the left-hand side.
- Products: Substances formed as a result of the reaction, written on the right-hand side.
- Information Provided: Equations reveal the formulas of reactants/products, their physical states (solid, liquid, gas), and special conditions like heat or catalysts.
- Limitations: Standard equations do not automatically reveal the speed of reaction, the exact concentration of reactants, or the heat changes involved unless specifically noted.
6. Balancing Equations
- Law of Conservation of Matter: In a chemical reaction, matter is neither created nor destroyed. Therefore, the total mass of reactants must equal the total mass of products.
- Balanced Equation: An equation where the number of atoms of each element is equal on both the reactant and product sides.
- Balancing Process: Equations are typically balanced using the hit and trial method, ensuring every atom on the left is accounted for on the right.
Quick Navigation:
| | | | |
1 / 1
Quick Navigation:
| | | | |