Quick Navigation:
| | | | |
Quick Navigation:
| | | | |
Hydrogen
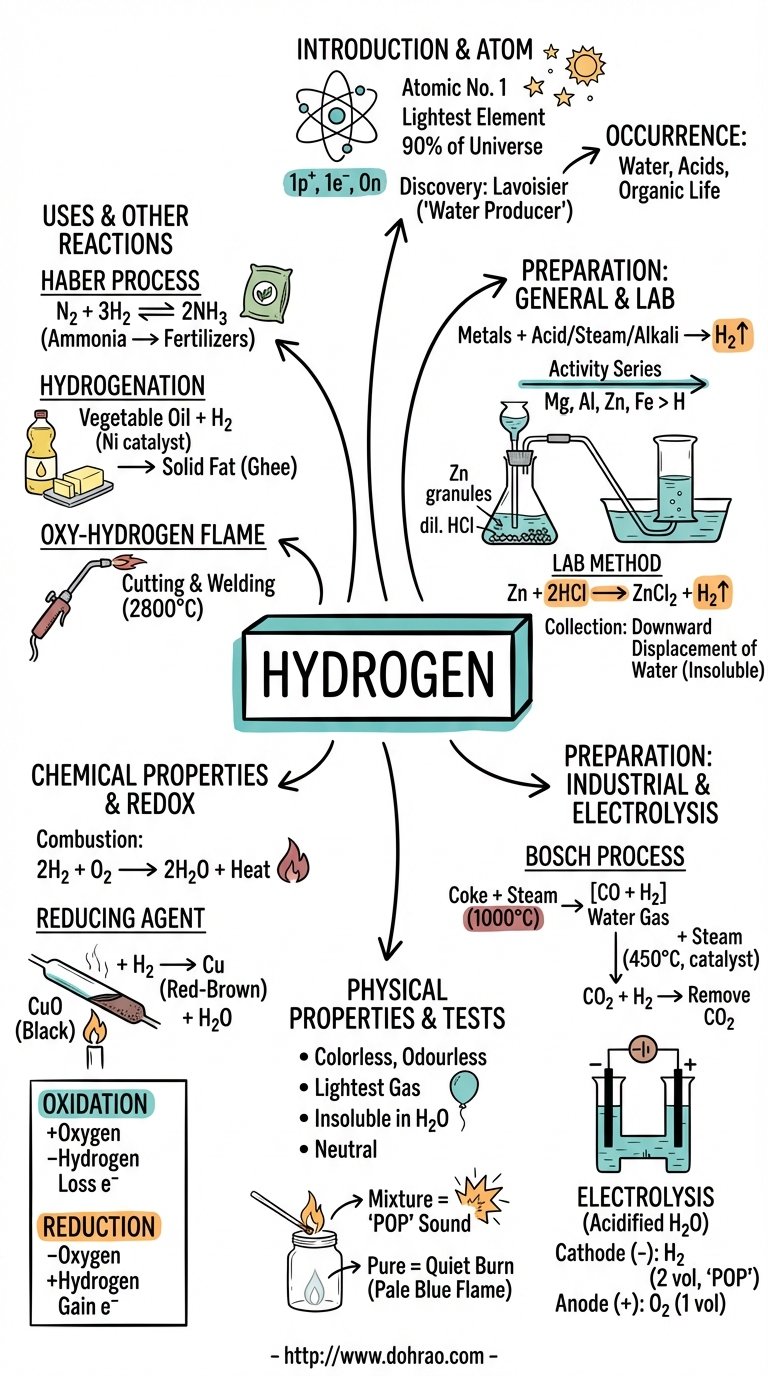
1. Introduction and Discovery
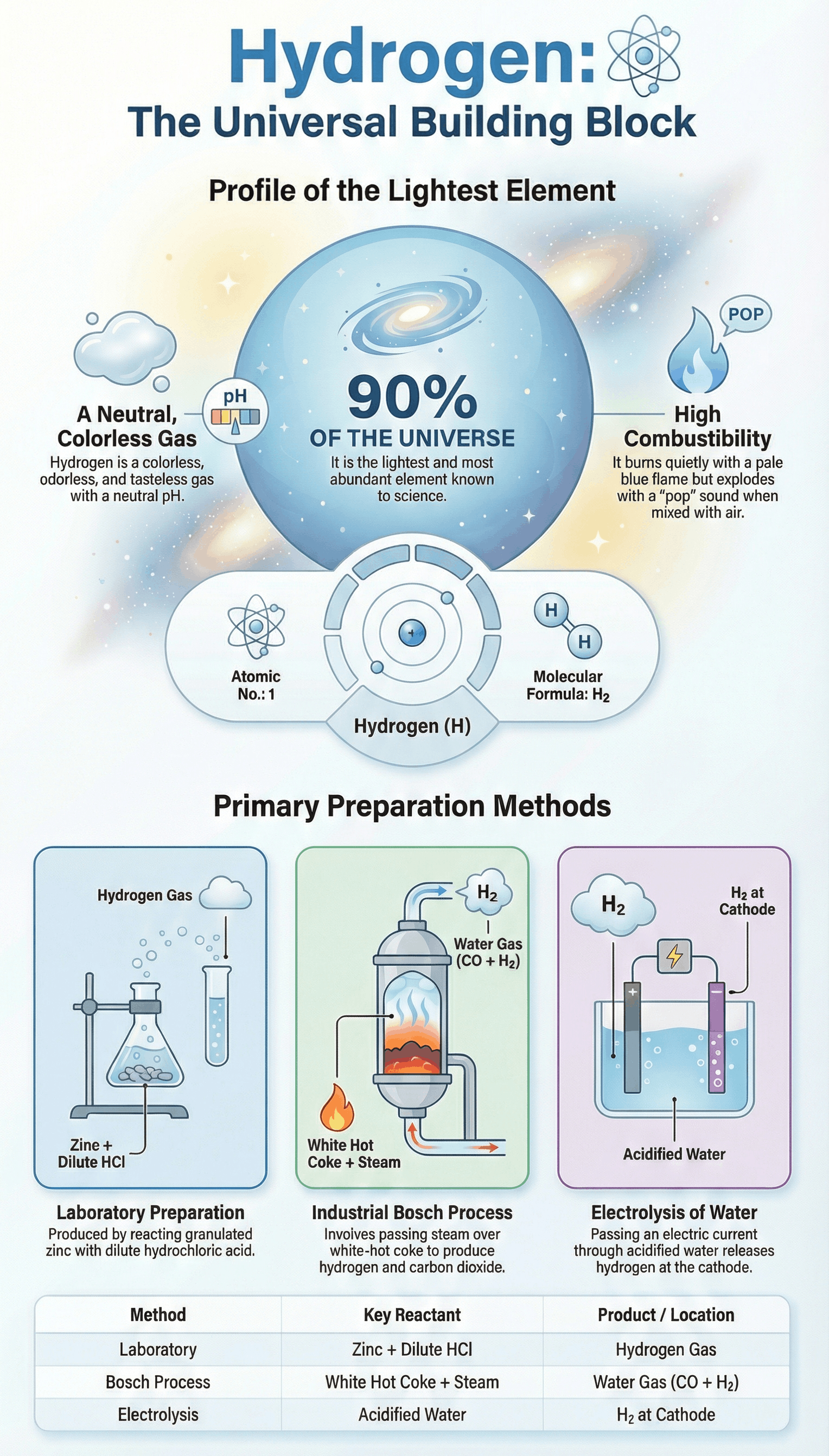
- Hydrogen (H) is the lightest and most abundant element in the universe, constituting about 90% of it.
- It has an Atomic Number of 1 and its molecular formula is H2.
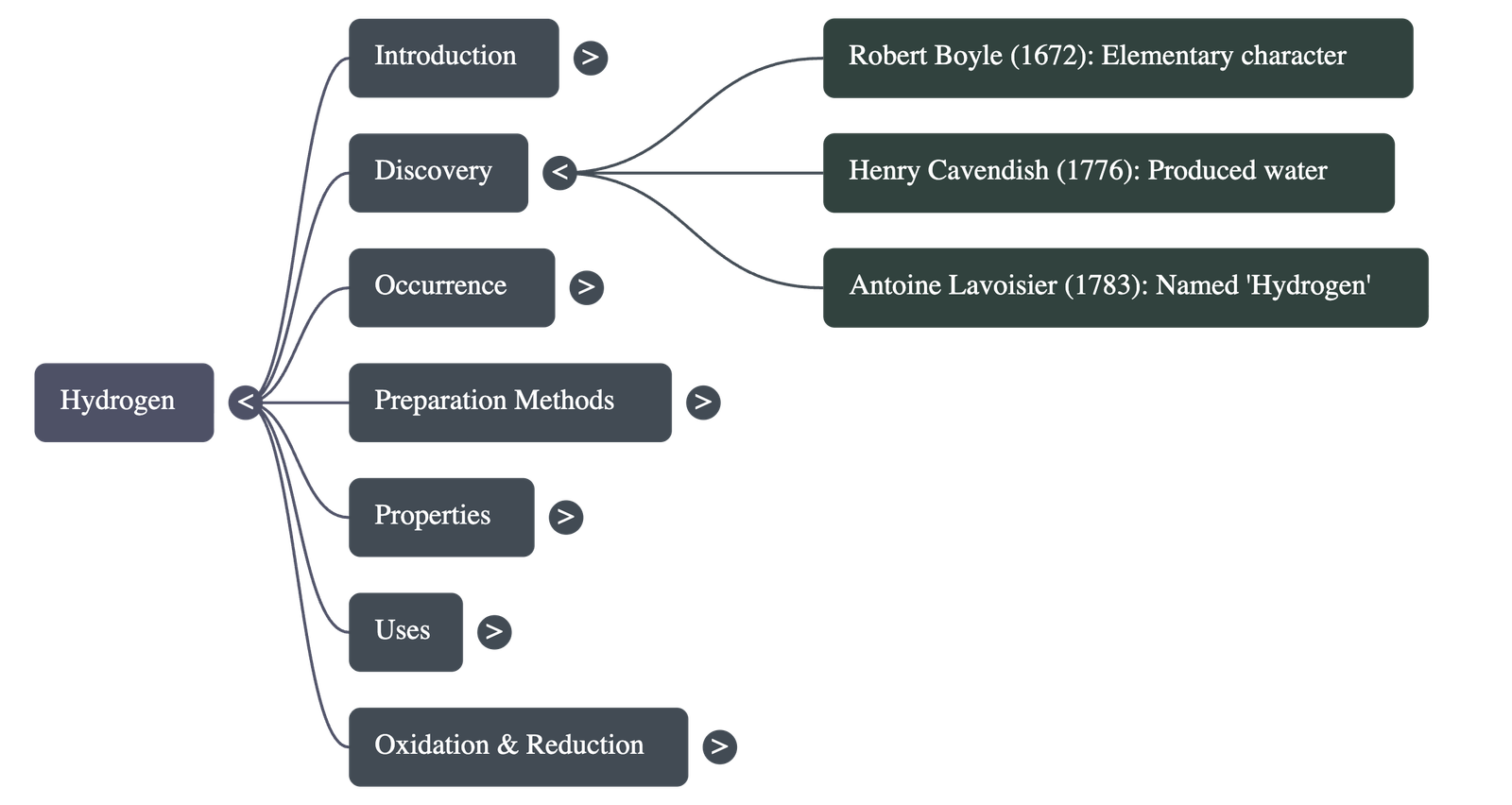
- Robert Boyle established its elementary character in 1672.
- Henry Cavendish discovered in 1776 that hydrogen burns in air to produce water.
- Antoine Lavoisier named the gas 'Hydrogen', meaning "water producer" (Greek: Hydro = water, gen = producer).
2. Occurrence
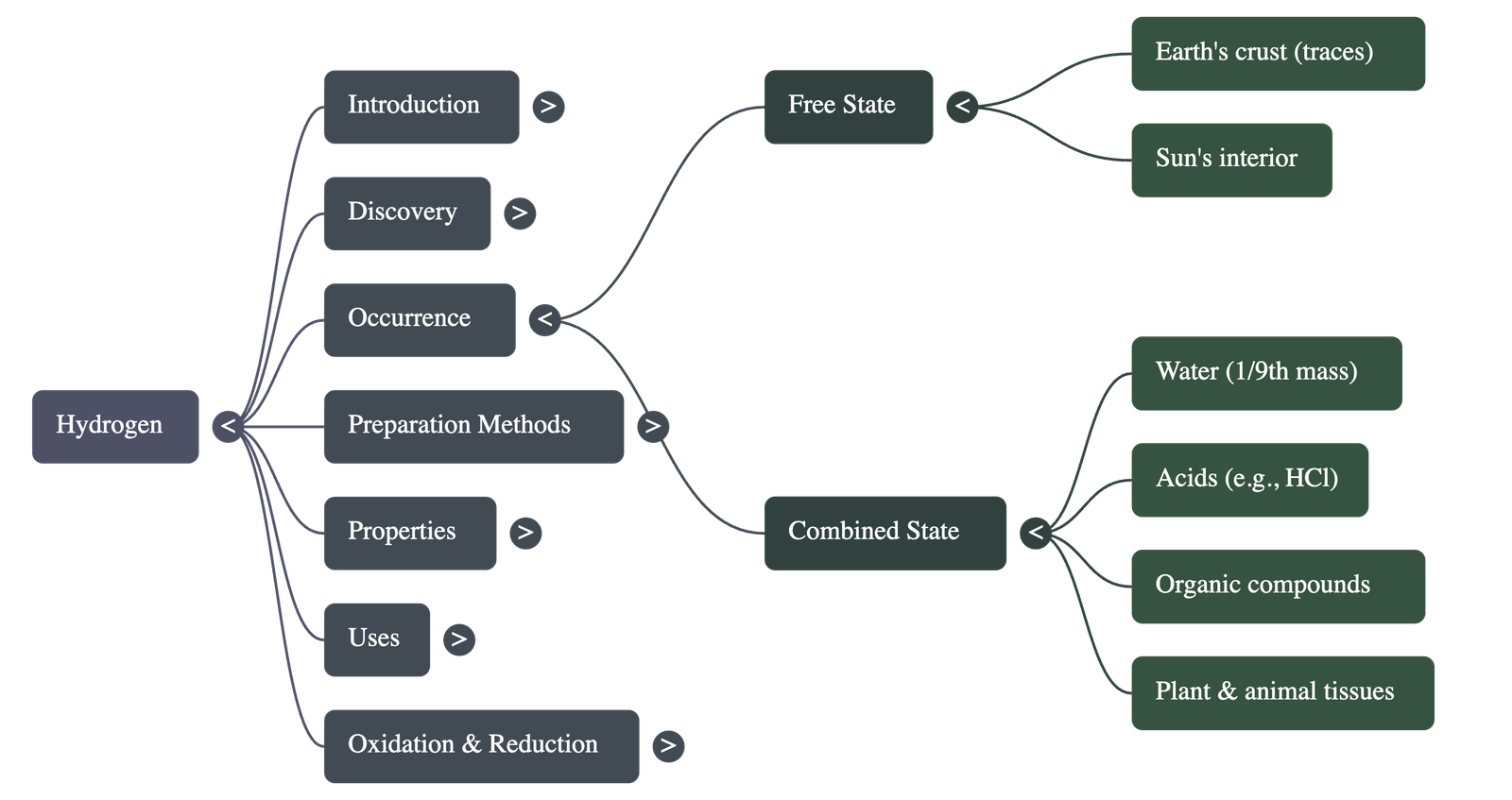
- Free State: Found in minute traces in the Earth's crust and atmosphere. It is the main constituent of the sun's interior, where it is converted into energy.
- Combined State: Found in water (constituting 1/9th by mass), acids (like HCl), and organic compounds (proteins, carbohydrates). It is also present in all plant and animal tissues.
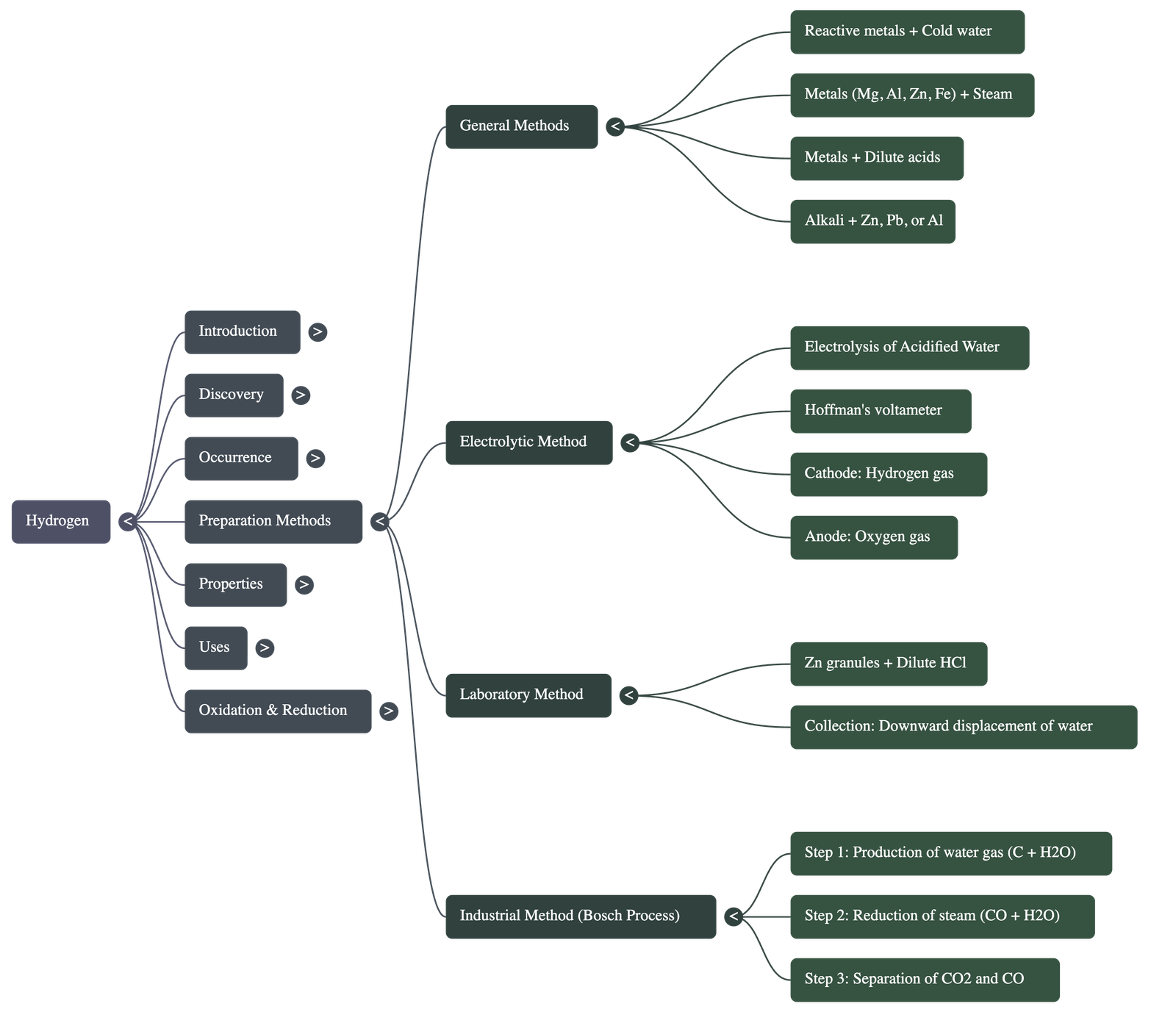
3. Preparation of Hydrogen
General Methods
- Reaction with Cold Water: Highly reactive metals like Potassium (K), Sodium (Na), and Calcium (Ca) react vigorously to liberate hydrogen and form metallic hydroxides.
- Reaction with Steam: Metals like Magnesium (Mg), Aluminium (Al), Zinc (Zn), and Iron (Fe) react with steam to form metallic oxides and hydrogen.
- Reaction with Dilute Acids: Metals above hydrogen in the activity series (Mg, Al, Zn, Fe) react with dilute HCl or H2SO4 to liberate hydrogen. Dilute nitric acid is not used because it is a strong oxidizing agent that turns the hydrogen into water.
- Reaction with Alkalis: Certain metals like Zinc, Lead, and Aluminium react with concentrated boiling alkalis (NaOH or KOH) to liberate hydrogen.
Electrolytic Method
- Hydrogen is prepared by the electrolysis of acidified water.
- Distilled water is a non-conductor; adding a few drops of acid provides free ions to conduct electricity.
- During the process, Hydrogen gas is collected at the Cathode (negative electrode) and Oxygen gas is collected at the Anode (positive electrode).
Laboratory Method
- Reactants: Granulated Zinc and dilute Hydrochloric acid.
- Procedure: Zinc granules are placed in a flask, and dil. HCl is added through a thistle funnel.
- Collection: Collected by the downward displacement of water because hydrogen is virtually insoluble in water. It is not collected by displacement of air because it forms an explosive mixture with air.
Industrial Method (Bosch Process)
- Step I: Production of Water Gas (CO + H2) by passing steam over white-hot coke at 1000°C.
- Step II: Reduction of steam by carbon monoxide at 450°C using Iron (III) oxide as a catalyst and Chromic oxide as a promoter.
- Step III: Separation of CO2 (dissolved in water under pressure) and unreacted CO (absorbed in ammoniacal cuprous chloride solution) to leave pure hydrogen.
4. Physical and Chemical Properties
Physical Properties
- Hydrogen is colorless, odourless, and tasteless.
- It is the lightest known gas (14.4 times lighter than air).
- It is neutral to litmus and only very slightly soluble in water.
Chemical Properties
- Combustibility: Pure hydrogen burns quietly with a pale blue flame to form water. A hydrogen-air mixture burns with a characteristic 'pop' sound.
- Reducing Agent: Hydrogen is a strong reducing agent. It removes oxygen from heated metallic oxides (like Copper Oxide) to form water and the free metal.
- Hydrogenation: The addition of hydrogen to organic compounds (like vegetable oils) in the presence of a catalyst like Nickel at 200°C to form solid fats (Vanaspati ghee).
- Reactions: It reacts with Chlorine to form Hydrogen Chloride, with Nitrogen (Haber Process) to form Ammonia, and with molten Sulphur to form Hydrogen Sulphide.
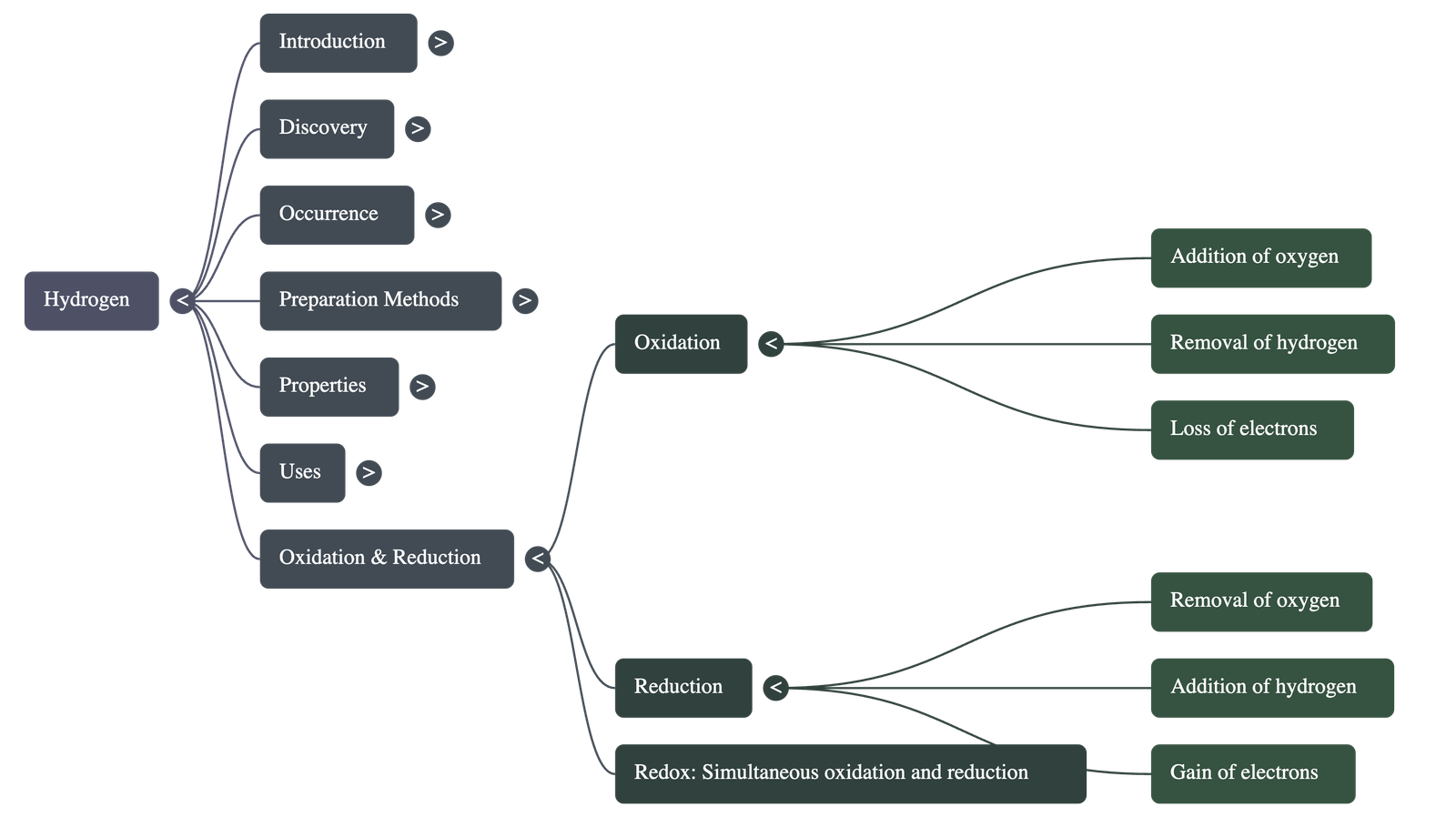
5. Oxidation and Reduction
- Oxidation: A chemical reaction involving the addition of oxygen, the removal of hydrogen, or the loss of electrons.
- Reduction: A chemical reaction involving the removal of oxygen, the addition of hydrogen, or the gain of electrons.
- Redox Reaction: A reaction where oxidation and reduction take place simultaneously.
- Oxidizing Agents: Substances that provide oxygen, remove hydrogen, or accept electrons (e.g., O2, Cl2, conc. H2SO4).
- Reducing Agents: Substances that gain oxygen, lose hydrogen, or donate electrons (e.g., H2, CO, C).
6. Uses of Hydrogen
- Used in Oxy-hydrogen torches for welding and cutting metals (reaches 2800°C).
- Used in the hydrogenation of vegetable oils to make solid fats.
- Used in the manufacture of chemicals like Ammonia, Nitric Acid, and fertilizers (Urea).
- Historically used in balloons, but now replaced by safer gases like Helium due to hydrogen's high inflammability.
Quick Navigation:
| | | | |
1 / 1
Quick Navigation:
| | | | |