Quick Navigation:
| | | | |
Quick Navigation:
| | | | |
Chemical Reactions
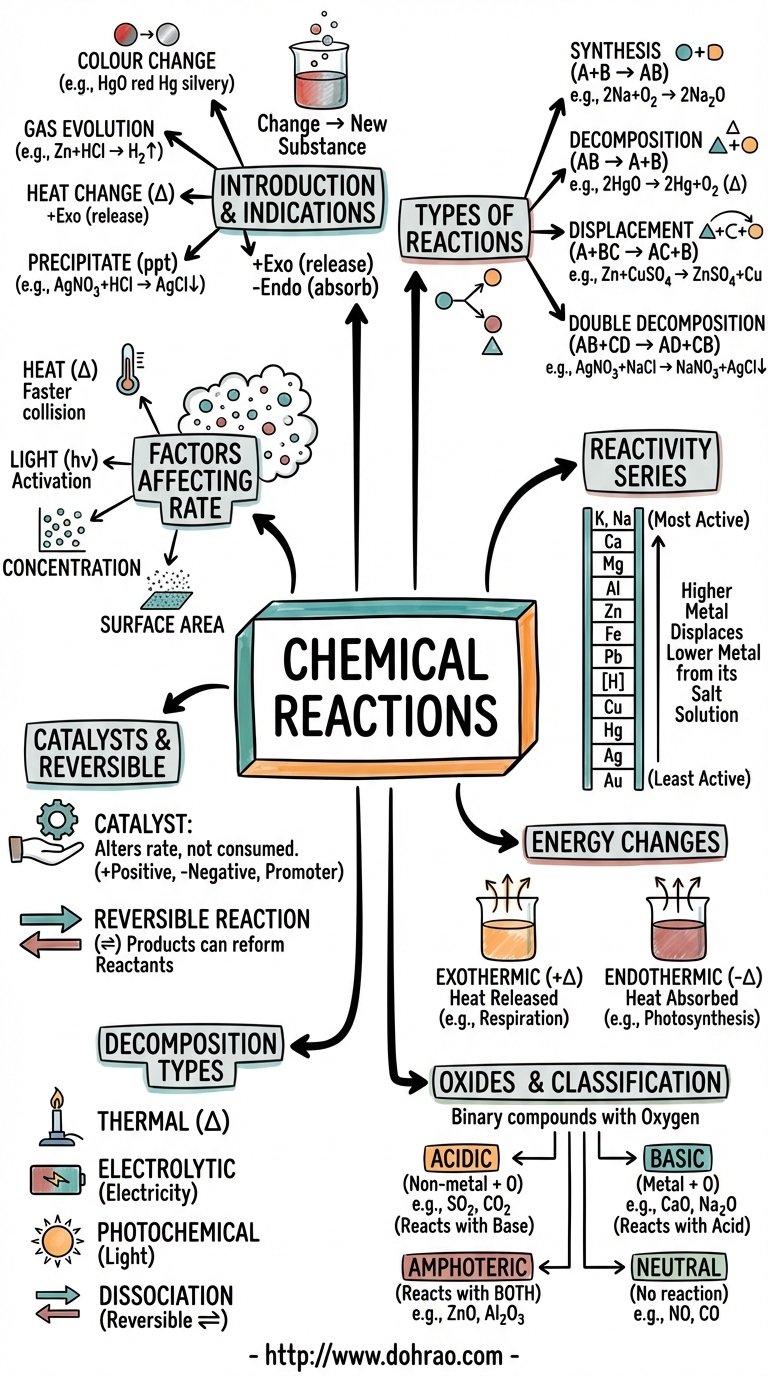
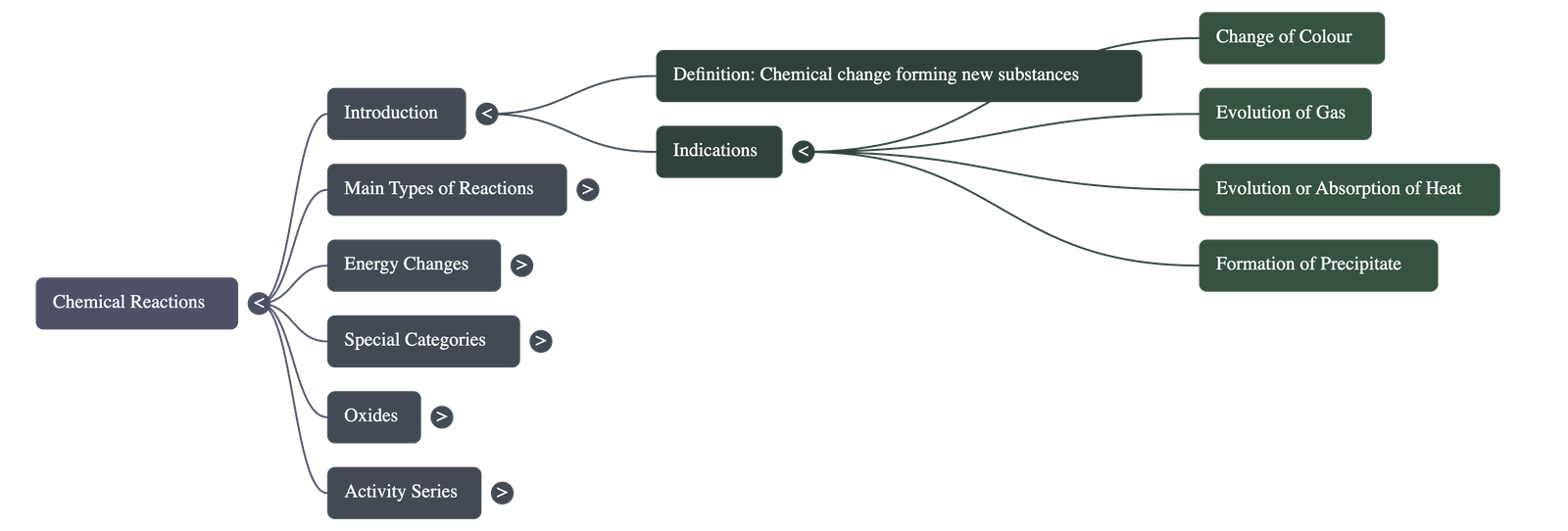
Introduction to Chemical Reactions
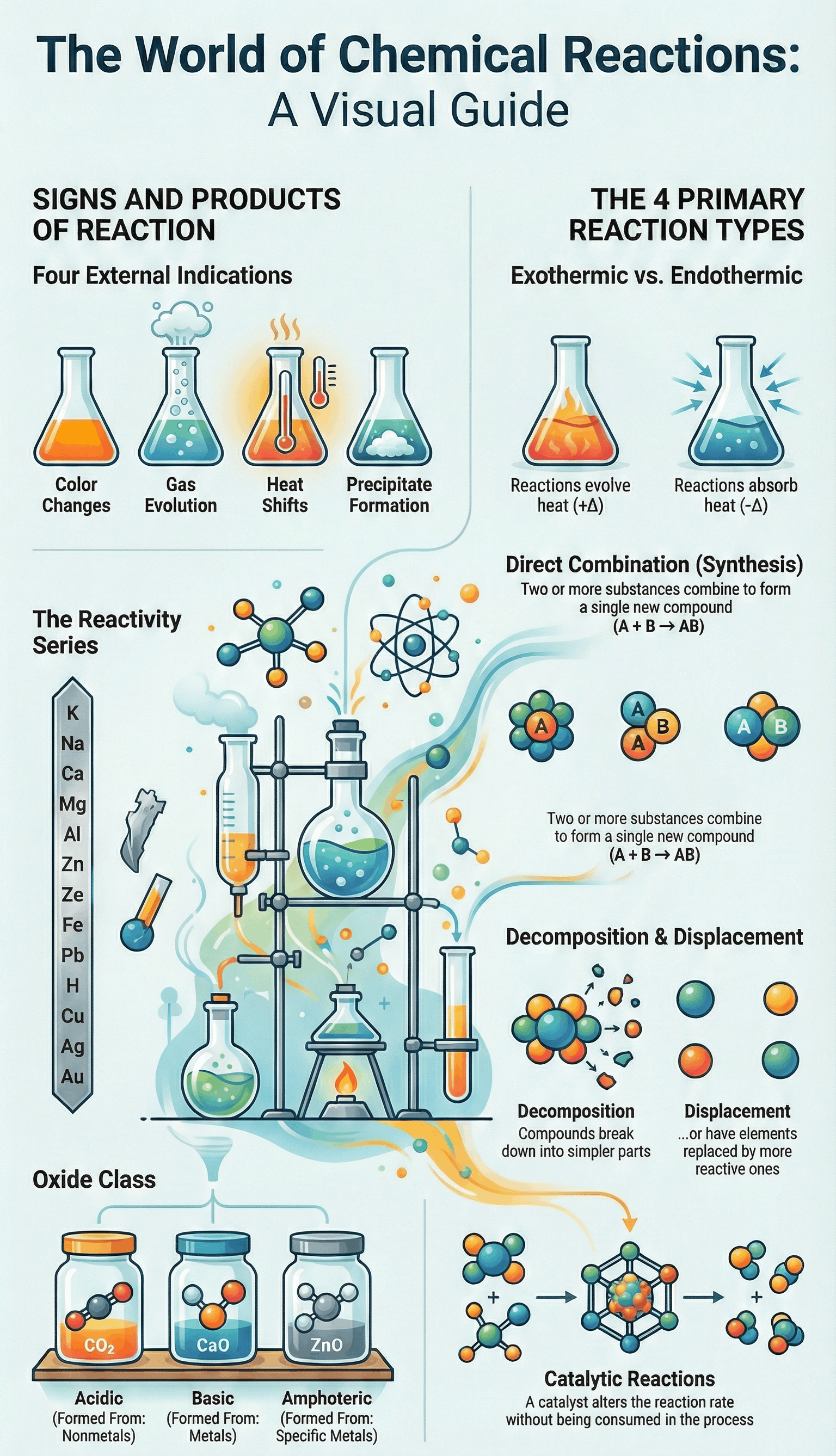
- Definition: A chemical reaction is a change in which matter transforms into one or more new substances.
- External Indications: Reactions are often identified by characteristics such as a change in color, evolution of a gas, evolution or absorption of heat, and the formation of an insoluble precipitate.
Main Types of Chemical Reactions
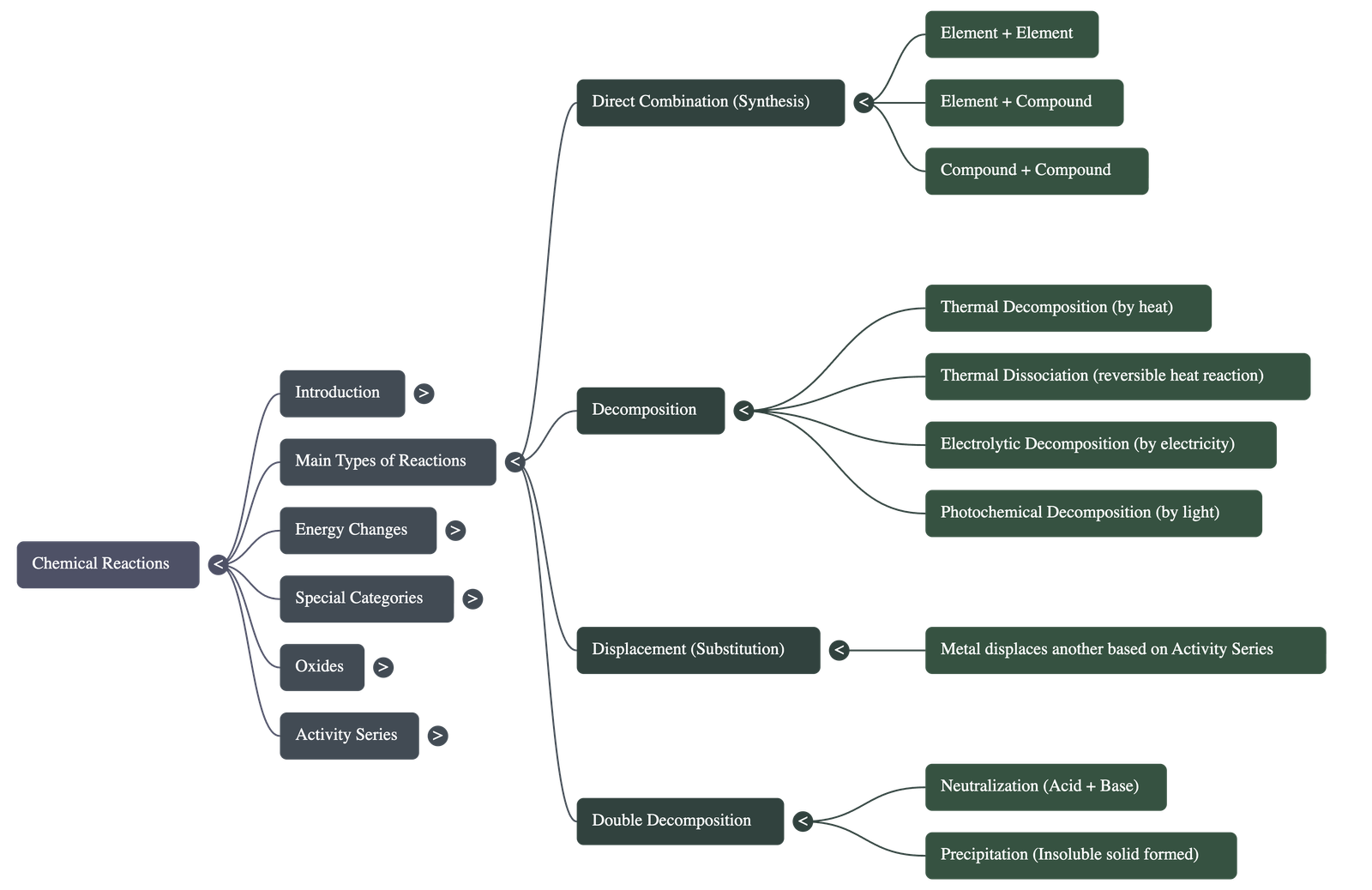
- Direct Combination (Synthesis): A reaction where two or more elements or compounds combine to form a single new compound.
- Decomposition Reaction: A process where a single chemical compound breaks down into two or more simpler substances (elements or compounds).
- Displacement Reaction (Substitution): A reaction in which a more reactive element replaces another element from its compound.
- Double Decomposition Reaction: A reaction where two reactants exchange their radicals to form two new compounds. This includes Neutralization (acid + base → salt + water) and Precipitation (formation of an insoluble solid).
Specific Decomposition Processes
- Thermal Decomposition: Decomposition brought about by the application of heat.
- Thermal Dissociation: A reversible decomposition reaction where a substance breaks down upon heating but recombines upon cooling.
- Electrolytic Decomposition: A reaction triggered by the passage of an electric current through a substance.
- Photochemical Decomposition: A reaction that occurs specifically in the presence of light, such as the decomposition of silver salts.
The Activity Series of Metals
- The activity series arranges metals in order of their reactivity, with the most active metals at the top and the least active at the bottom.
- Metals higher in the series displace those lower in the series from their salt solutions.
- The series determines how metals react with water, acids, and air; for example, metals above hydrogen displace it from dilute acids.
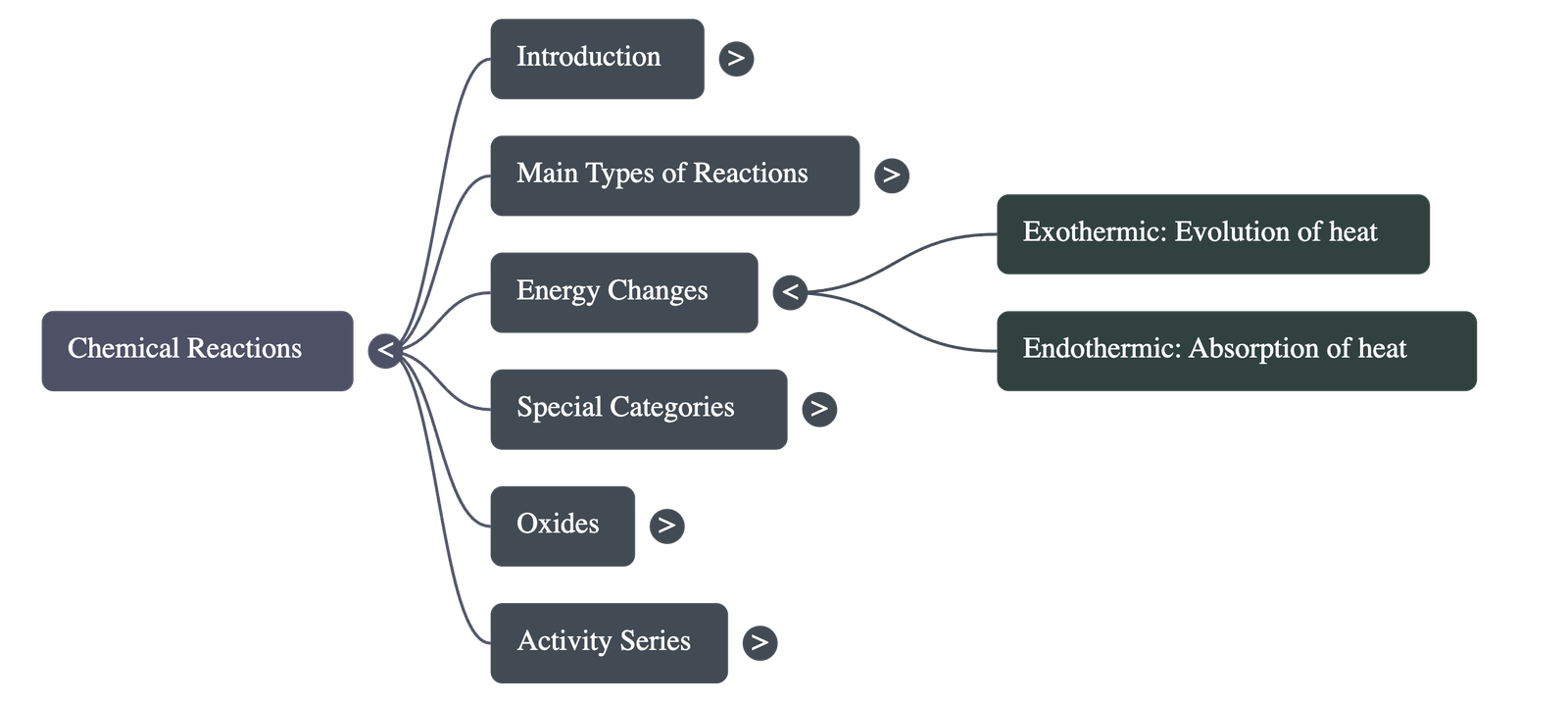
Energy Changes in Reactions
- Exothermic Reactions: Chemical reactions that proceed with the evolution of heat energy.
- Endothermic Reactions: Chemical reactions that proceed with the absorption of heat energy.
Catalytic and Reversible Reactions
- Catalytic Reaction: A reaction where a catalyst is used to alter the rate of the reaction without being consumed itself.
- Promoters: Substances added to a catalyst to increase its efficiency.
- Enzymes: Biological catalysts found in the human body that accelerate biochemical reactions.
- Reversible Reaction: A reaction where the products can react together to reform the original reactants under specific conditions.
Classification of Oxides
Oxides are binary compounds of an element with oxygen, classified into four main types:
- Acidic Oxides: Non-metal oxides that react with water to form acids and with bases to form salt and water.
- Basic Oxides: Metal oxides that react with water to form bases and with acids to form salt and water.
- Amphoteric Oxides: Metal oxides (like ZnO and PbO) that react with both acids and bases to give salt and water.
- Neutral Oxides: Oxides that do not exhibit acidic or basic properties and do not react with acids or bases.
Quick Navigation:
| | | | |
1 / 1
Quick Navigation:
| | | | |