Quick Navigation:
| | | | |
Quick Navigation:
| | | | |
Elements, Compounds & Mixtures
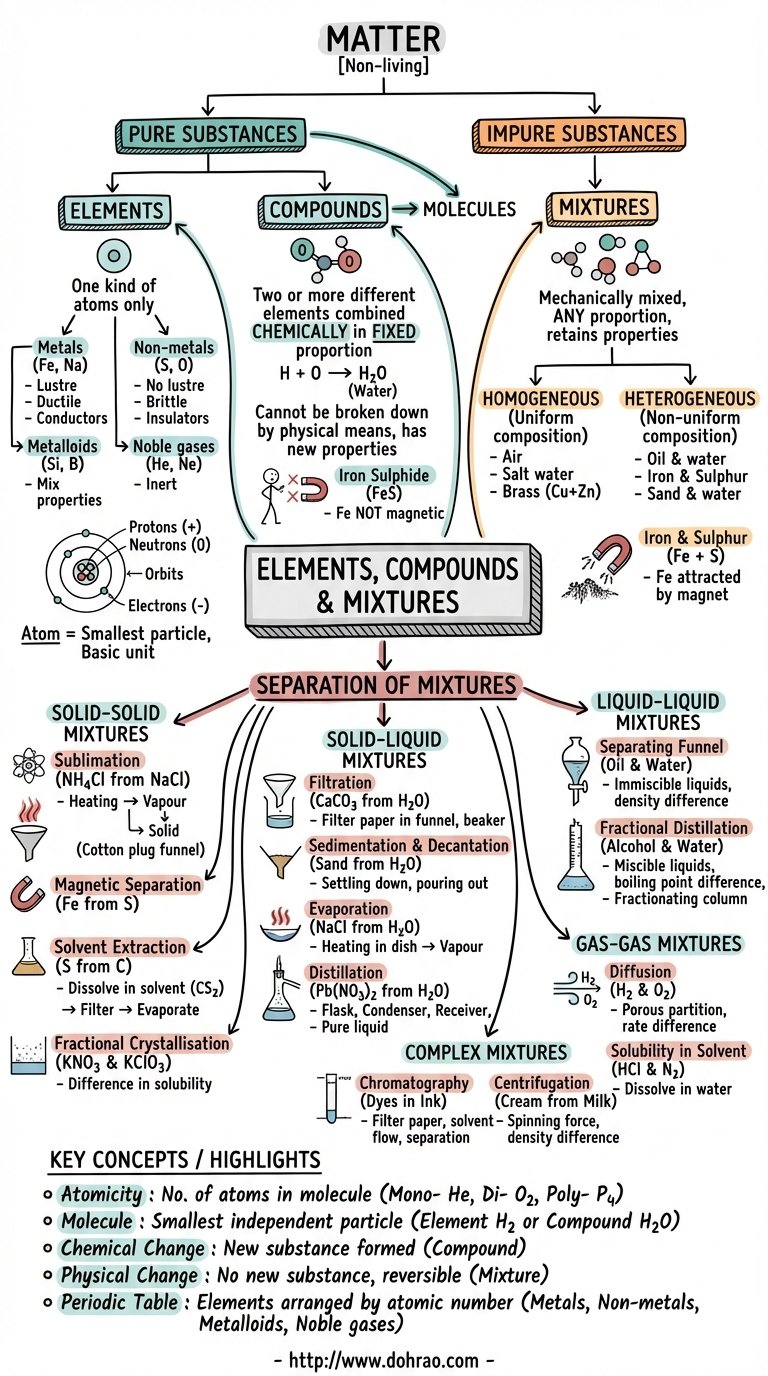
1. Classification of Matter
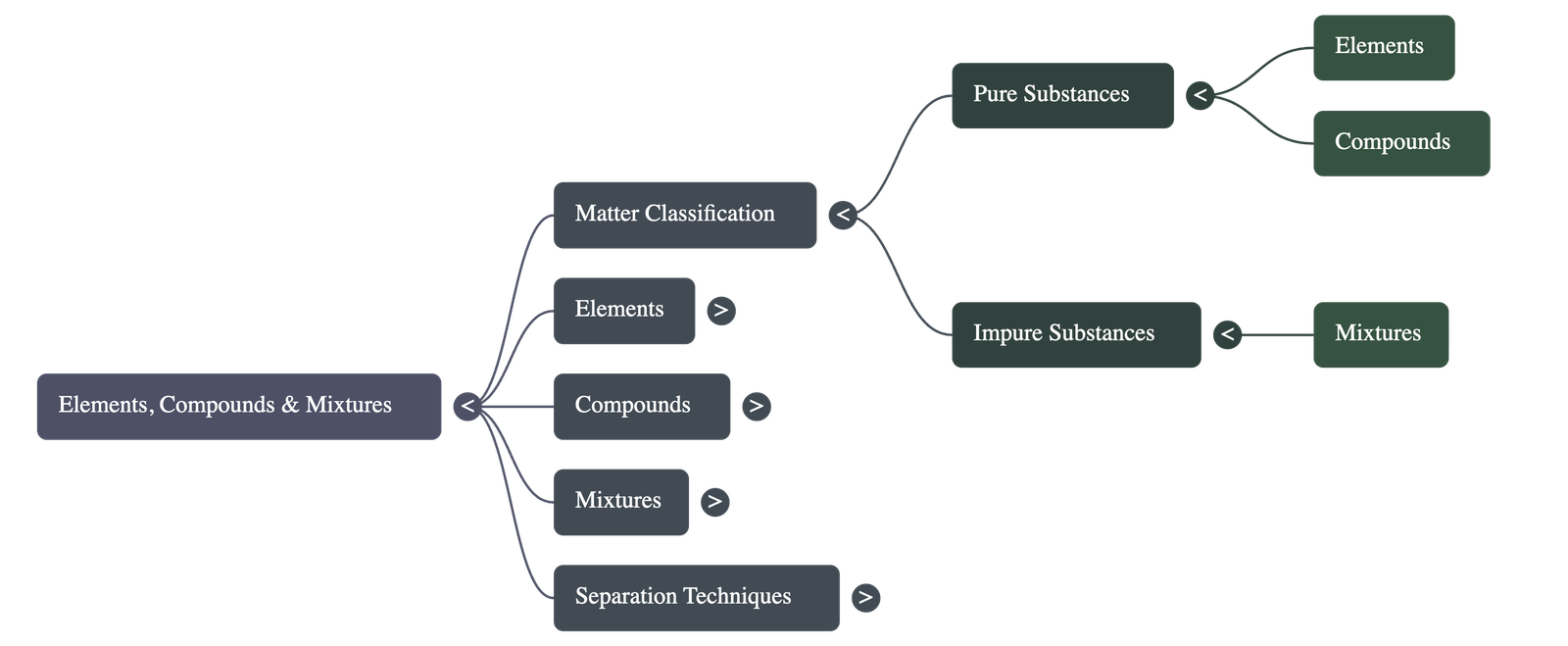
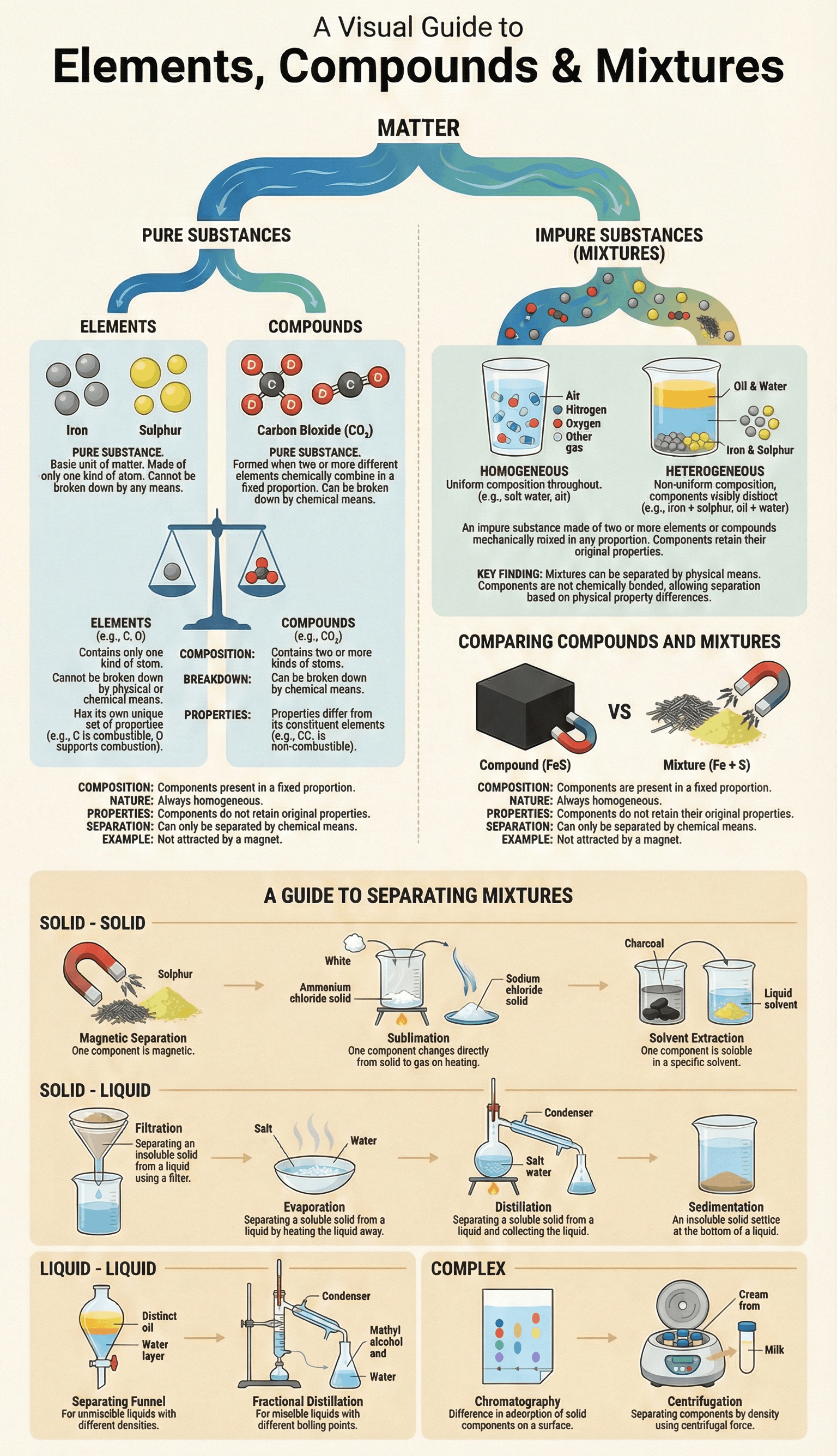
- Matter is broadly classified into two categories: Pure Substances and Impure Substances.
- Pure Substances include Elements (like Iron and Sulphur) and Compounds (like Iron Sulphide).
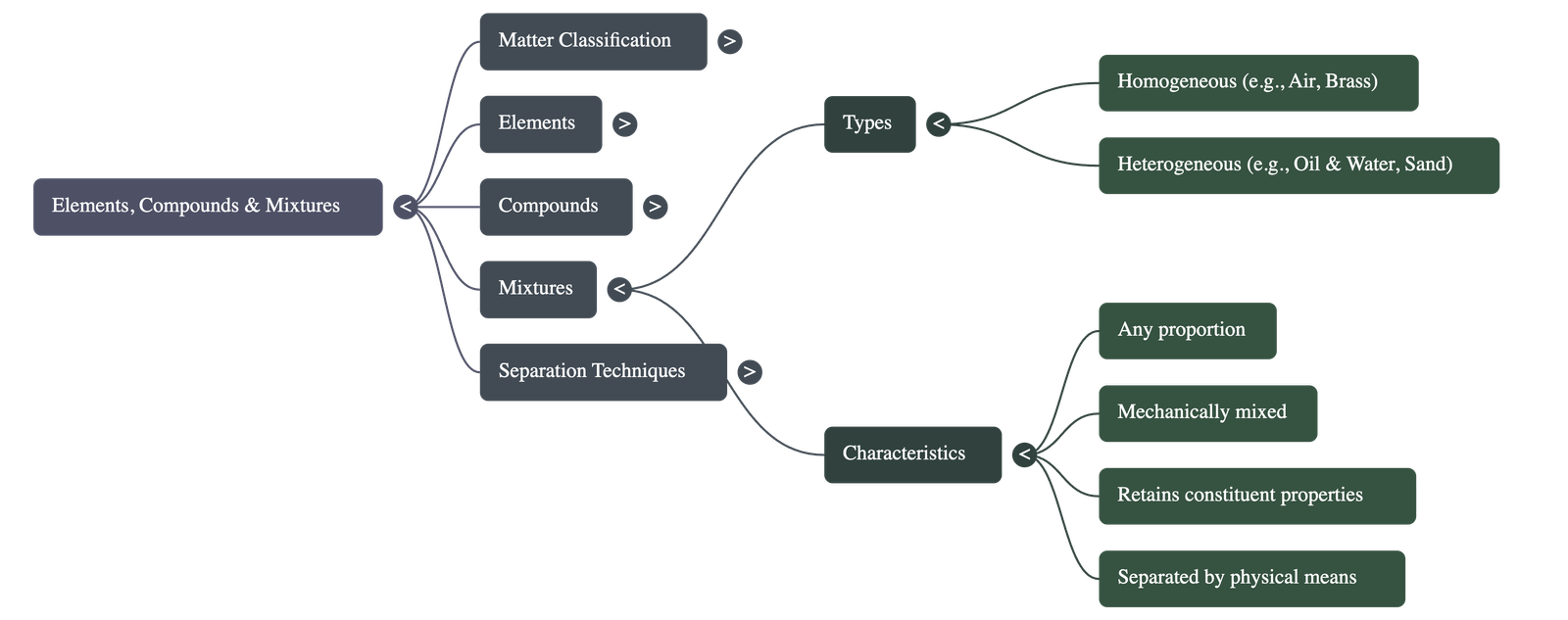
- Impure Substances are known as Mixtures, which can be homogeneous (like Air) or heterogeneous (like Oil and Water).
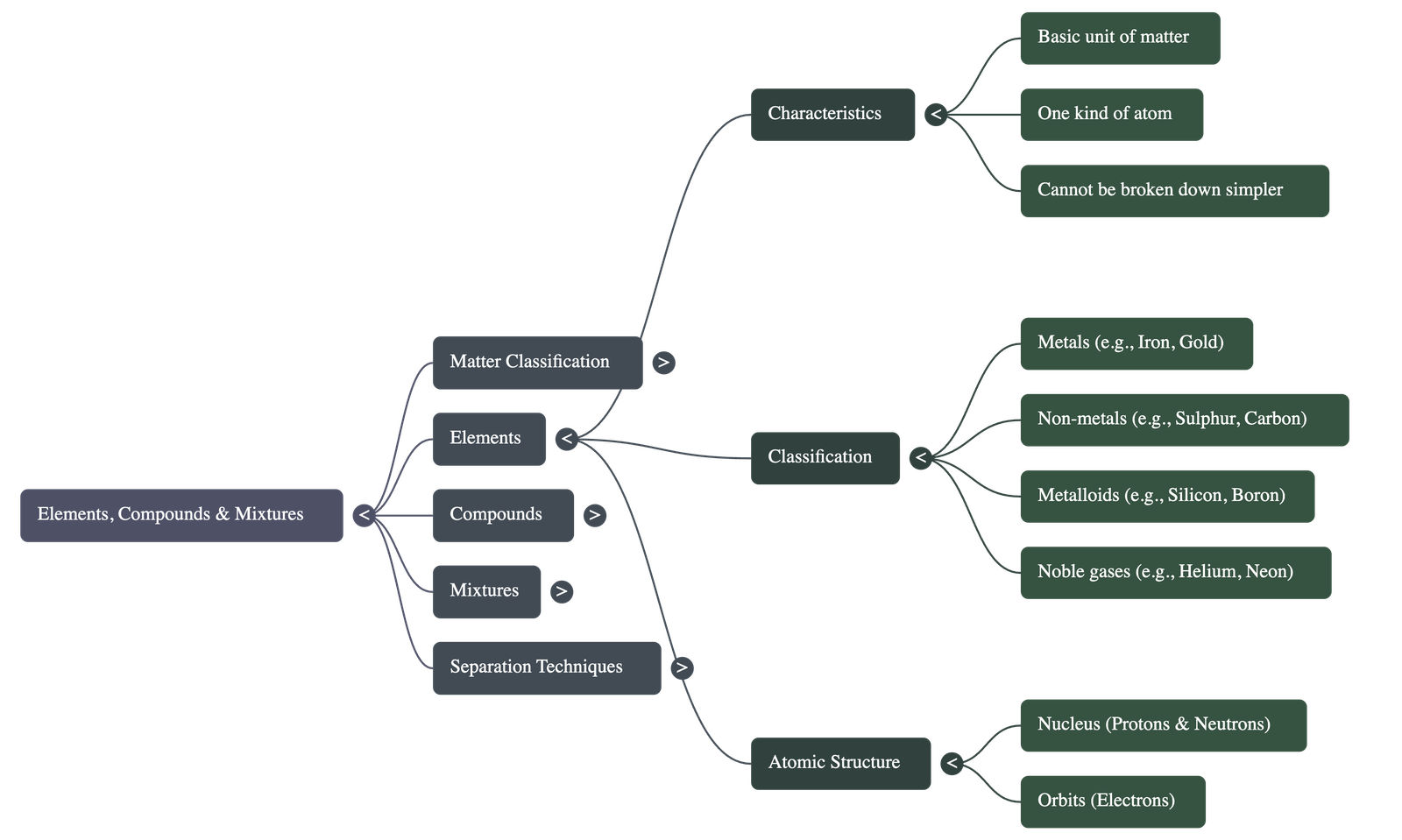
2. Elements and Atoms
- An Element is the basic unit of matter, made up of only one kind of atom, and cannot be broken down into simpler substances.
- Atoms are the smallest particles of an element. They consist of a Nucleus (containing positive protons and neutral neutrons) surrounded by Orbits where negative electrons revolve.
- Elements are classified into Metals (lustrous, malleable, ductile, good conductors), Non-metals (non-lustrous, poor conductors), Metalloids (showing properties of both), and Noble Gases (chemically inactive).
3. Molecules and Atomicity
- A Molecule is the smallest particle of a substance that can normally exist independently while retaining its physical and chemical properties.
- Atomicity refers to the number of atoms in a molecule: Monoatomic (1 atom, e.g., Helium), Diatomic (2 atoms, e.g., Oxygen), or Polyatomic (more than 2 atoms, e.g., Ozone).
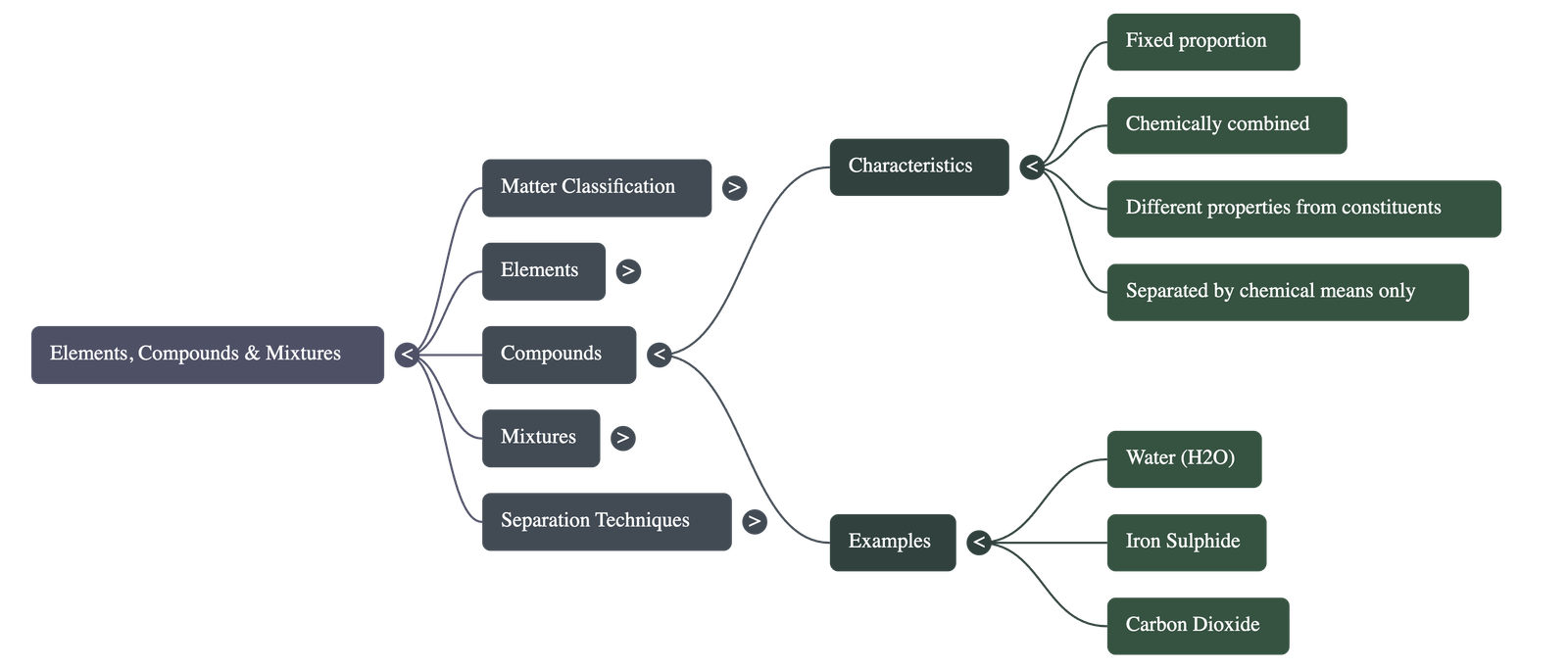
4. Compounds
- A Compound is a pure substance formed by the chemical combination of two or more different elements in a fixed proportion.
- Components of a compound lose their original properties; for example, combustible Carbon and life-supporting Oxygen combine to form Carbon Dioxide, which is a non-supporter of combustion.
- The constituents of a compound can only be separated by chemical means.
5. Mixtures
- A Mixture is formed when two or more substances are mechanically mixed in any proportion without a chemical reaction.
- Constituents of a mixture retain their original properties (e.g., in an iron and sulphur mixture, iron is still attracted by a magnet).
- Homogeneous mixtures have a uniform composition throughout (e.g., Salt solution), while Heterogeneous mixtures do not (e.g., Sand and water).
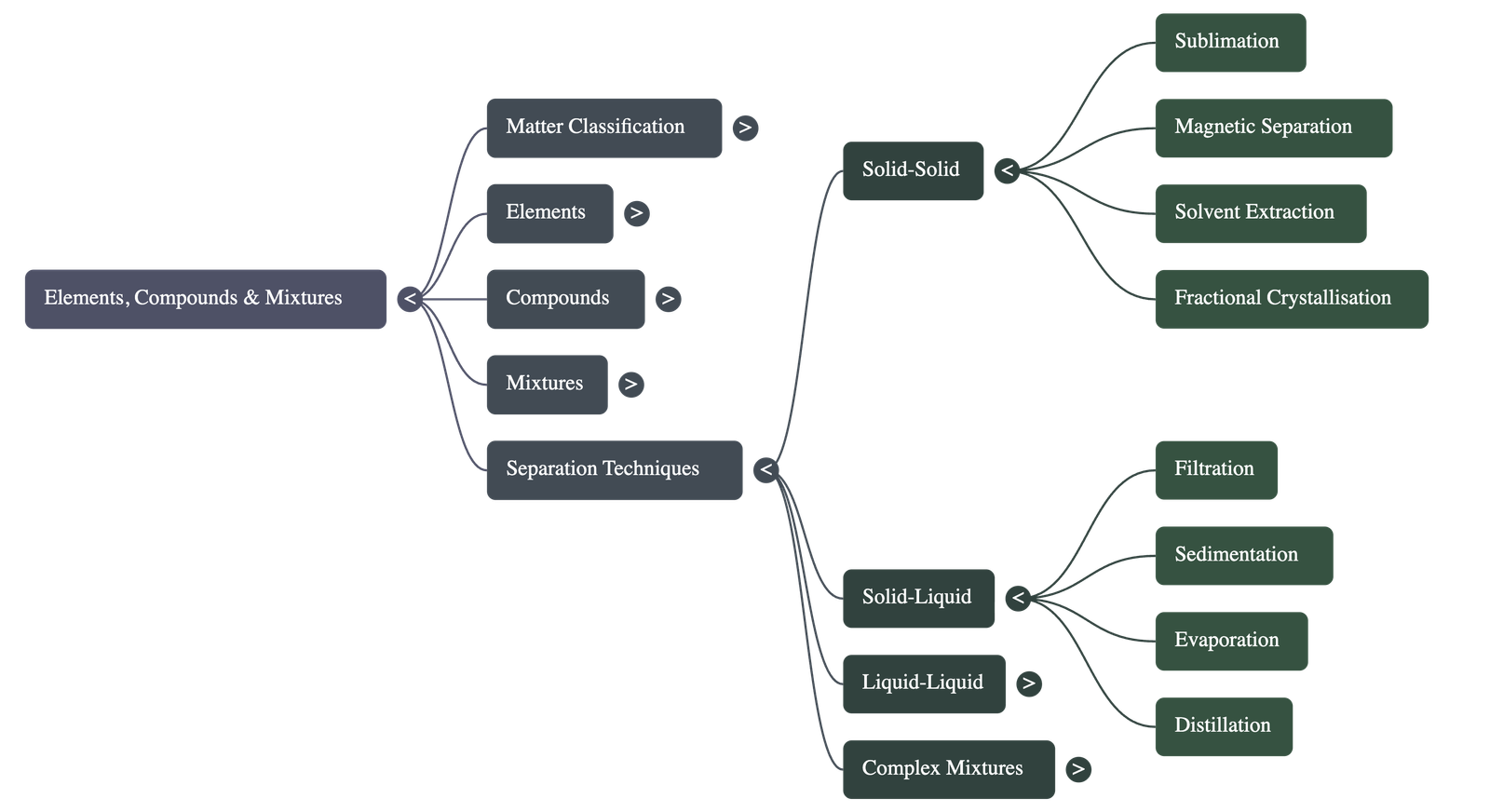
6. Separation Techniques
- Solid-Solid Separation: Includes Sublimation (for substances like Ammonium Chloride), Magnetic Separation (using magnets for iron), Solvent Extraction (based on solubility), and Fractional Crystallisation.
- Solid-Liquid Separation: Includes Filtration (using filter paper), Sedimentation/Decantation (settling of solids), Evaporation (recovering soluble solids), and Distillation (recovering both solid and liquid).
- Liquid-Liquid Separation: Separating Funnels are used for immiscible liquids (like Oil and Water), while Fractional Distillation is used for miscible liquids with different boiling points.
- Complex Mixtures: Chromatography is used to separate different dyes in ink based on adsorption, and Centrifugation uses centrifugal force to separate components based on density (e.g., cream from milk).
Quick Navigation:
| | | | |
1 / 1
Quick Navigation:
| | | | |
Quick Navigation:
| | | | |