Quick Navigation:
| | | | |
Quick Navigation:
| | | | |
Point-wise summary of the chapter on Heat Transfer:
1. Nature of Heat and Matter
- Matter is composed of tiny particles called molecules that are in constant motion and possess both kinetic energy (due to motion) and potential energy (due to attractive forces).
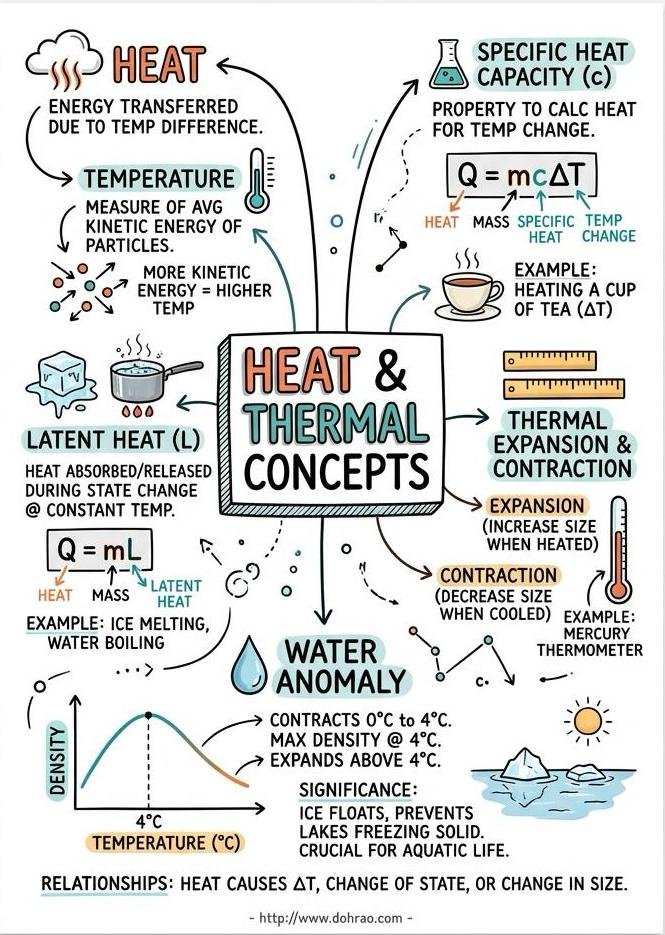
- Heat is defined as the internal energy of a substance, representing the sum of the total internal kinetic and potential energy of its molecules.
- Heat is measured in joules (J), though calories (cal) are also commonly used (1 cal ≈ 4.2 J).
2. Effects of Heat
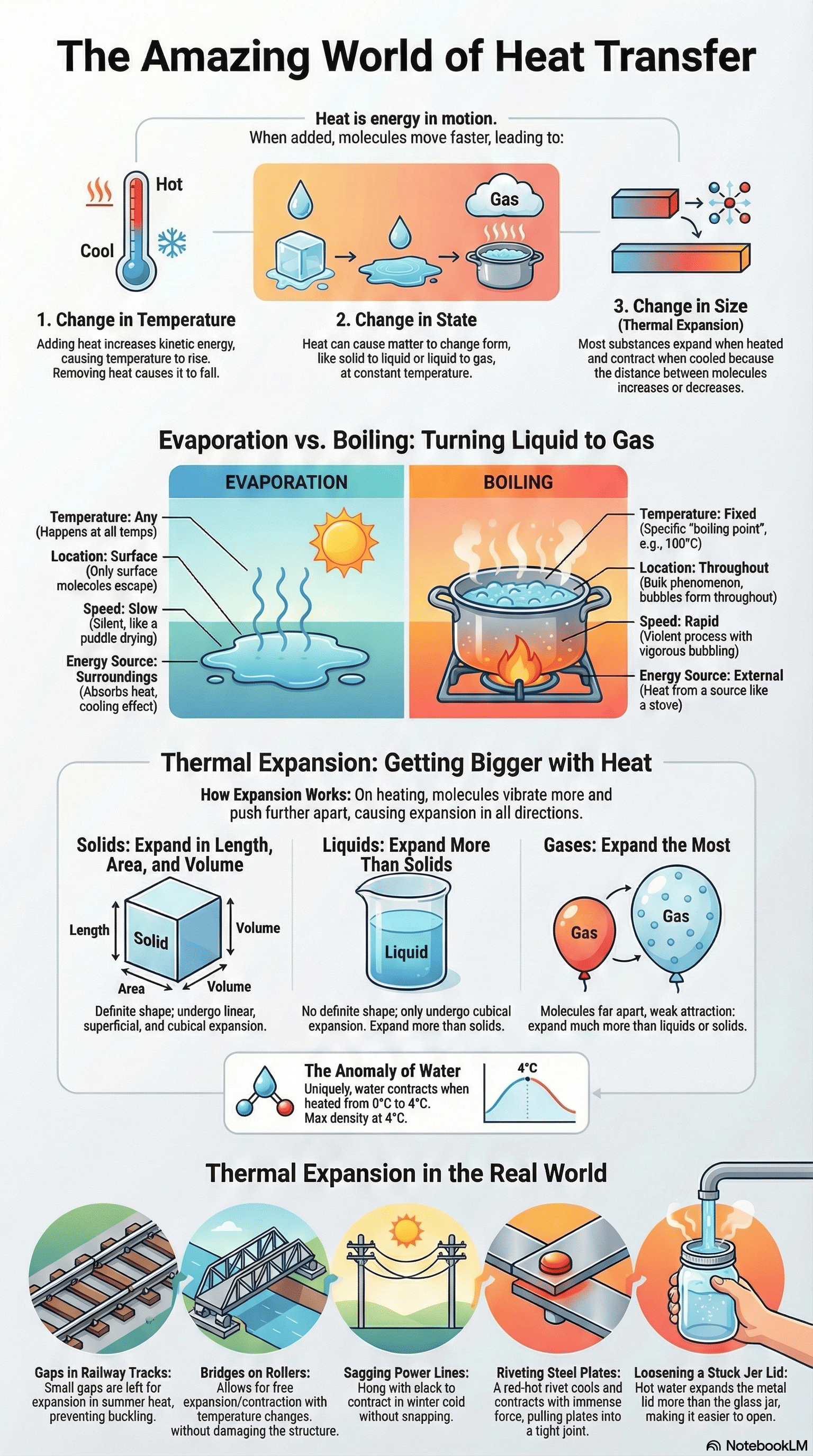
- Change in Temperature: Heating increases the average kinetic energy of molecules, causing the temperature to rise, while cooling decreases it.
- Change in State: Matter can change between solid, liquid, and gas through processes like melting, freezing, vaporization, condensation, and sublimation.
- Change in Size: Substances generally expand on heating and contract on cooling, a phenomenon known as thermal expansion.
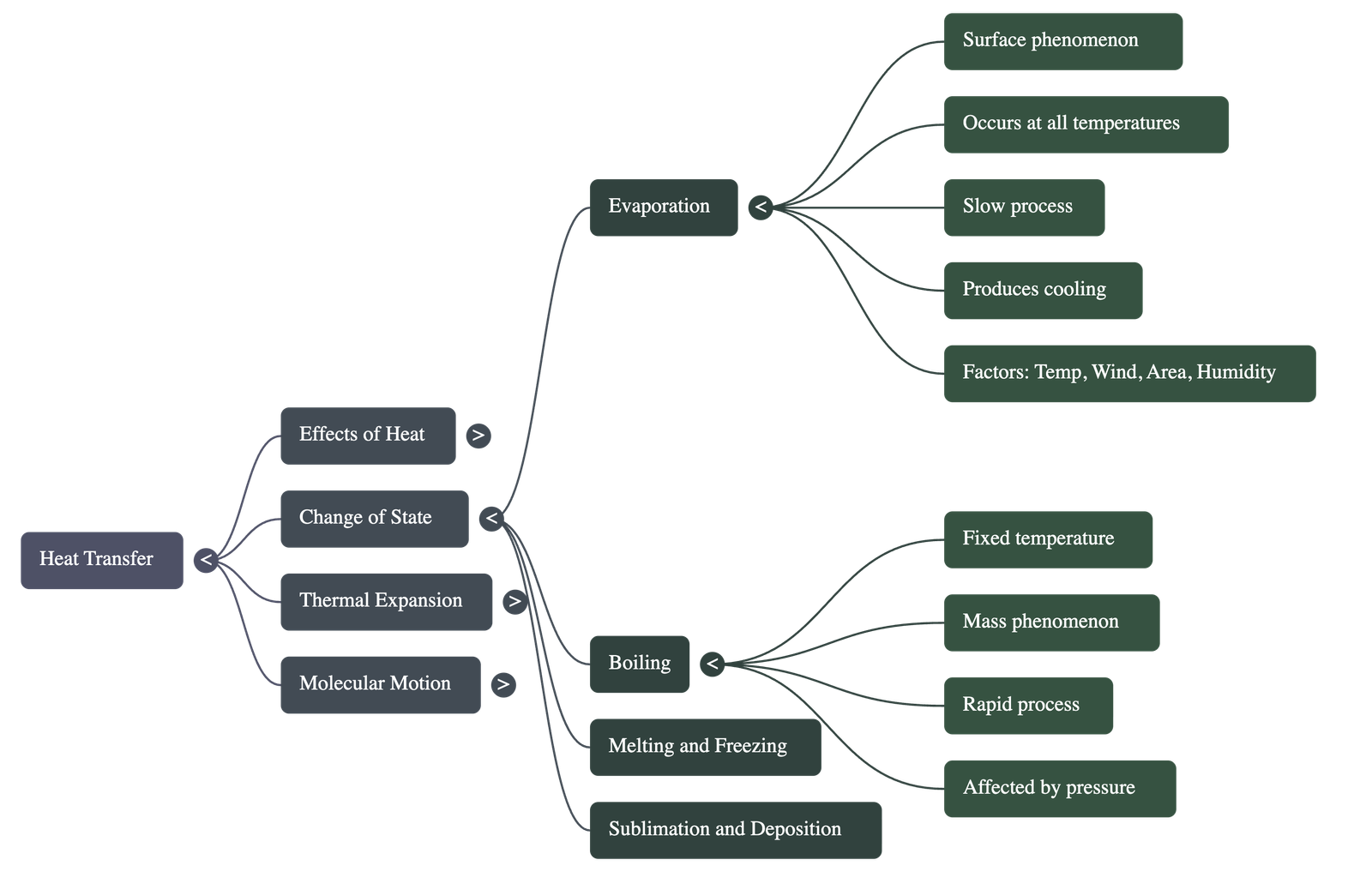
3. Vaporization: Evaporation vs. Boiling
- Evaporation is a slow surface phenomenon that occurs at all temperatures; molecules with high kinetic energy escape from the surface, drawing heat from the surroundings and causing a cooling effect.
- Boiling is a rapid process that occurs throughout the entire mass of a liquid at a fixed temperature called the boiling point.
- The rate of evaporation increases with higher temperatures, increased surface area, and blowing air, but decreases with higher humidity.
- Boiling point is affected by pressure; it increases with higher pressure (e.g., in a pressure cooker) and decreases with lower pressure (e.g., on mountains).
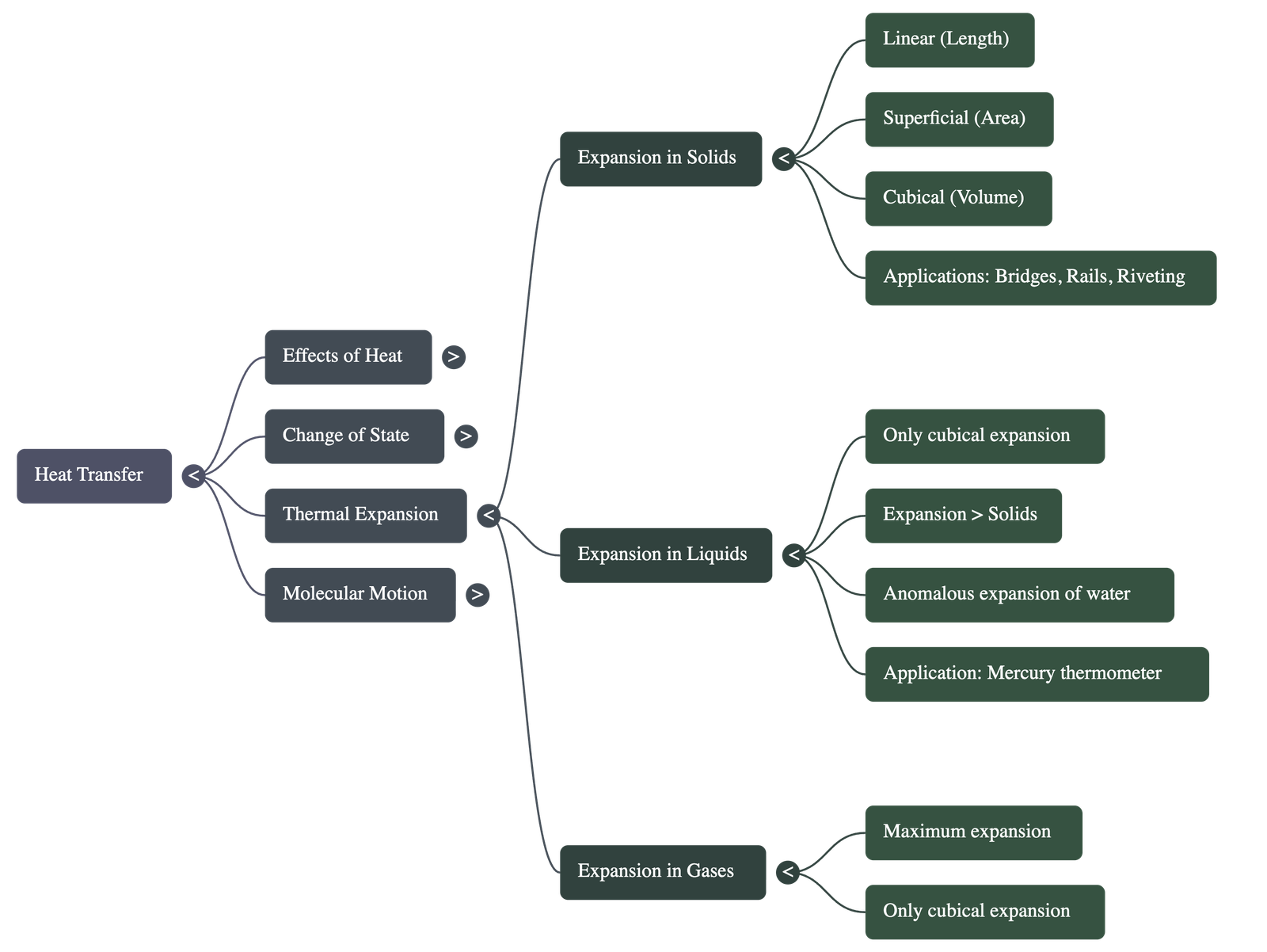
4. Thermal Expansion in Solids
- Solids undergo three types of expansion: linear (length), superficial (area), and cubical (volume).
- The increase in length depends on the original length, the rise in temperature, and the material of the solid.
- The relationship between the coefficients of linear ($\alpha$), superficial ($\beta$), and cubical ($\gamma$) expansion is expressed as $\alpha : \beta : \gamma = 1 : 2 : 3$.
- Daily Life Applications: Gaps are left between railway tracks and one end of a steel bridge is kept on rollers to allow for expansion/contraction without causing structural damage.
5. Thermal Expansion in Liquids and Gases
- Liquids and gases do not have a definite shape, so they only exhibit cubical expansion.
- Liquids expand more than solids, and gases expand much more than liquids when heated to the same temperature.
- Thermal expansion of liquids is utilized in the functioning of mercury thermometers.
- Anomalous Expansion of Water: Unlike most substances, water contracts when heated from $0^\circ C$ to $4^\circ C$, reaching its maximum density at $4^\circ C$.
6. Density and Temperature
- As a substance is heated, its volume increases while its mass remains constant, leading to a decrease in density.
- In water, because it contracts between $0^\circ C$ and $4^\circ C$, its density actually increases during that specific temperature range before decreasing as it is heated further.
Analogy for Thermal Expansion: Imagine a crowded room where people are standing still; they take up a specific amount of space. If those people suddenly start dancing energetically (like molecules gaining heat), they will need more room to move their arms and legs without hitting each other, causing the "group" to spread out and occupy a larger area.
Quick Navigation:
| | | | |
1 / 1
Quick Navigation:
| | | | |