Quick Navigation:
| | | | |
Point-wise summary of the key concepts regarding matter:
1. Definition and Composition of Matter
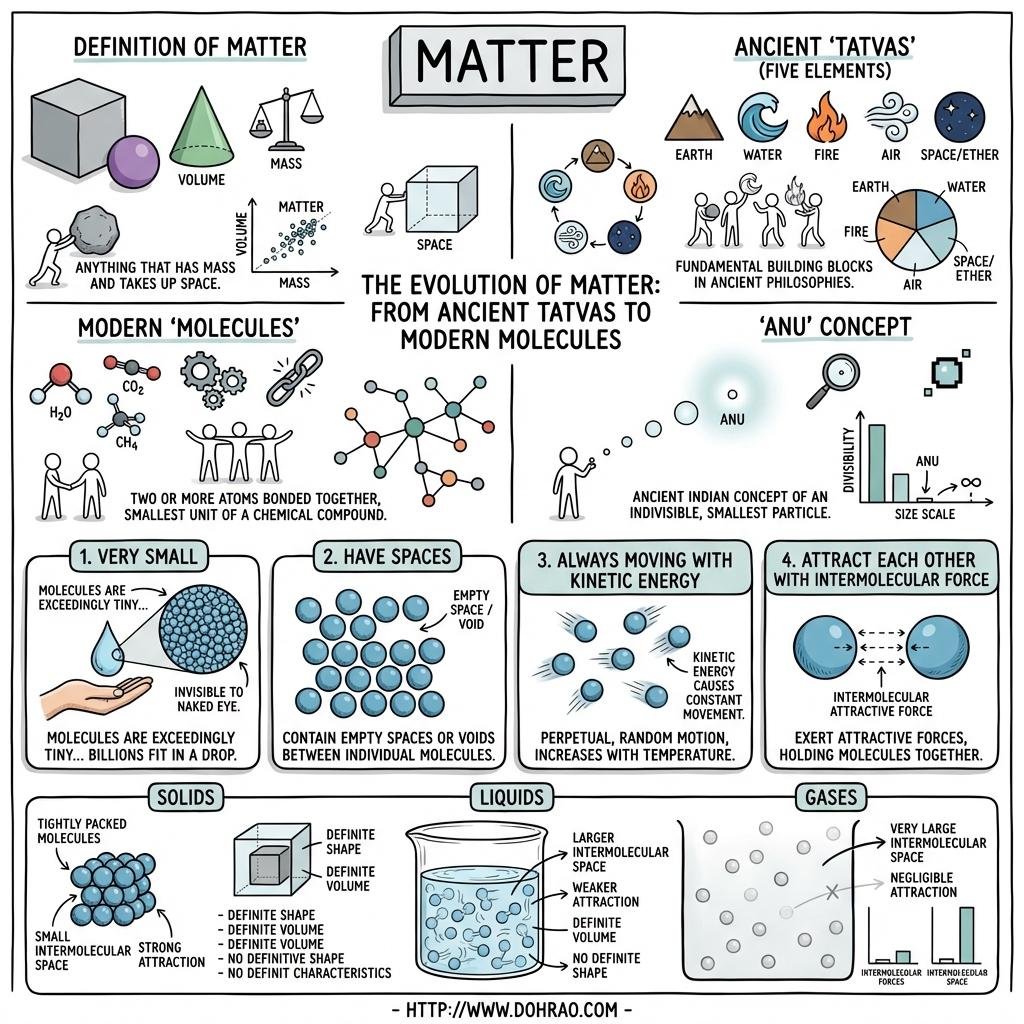
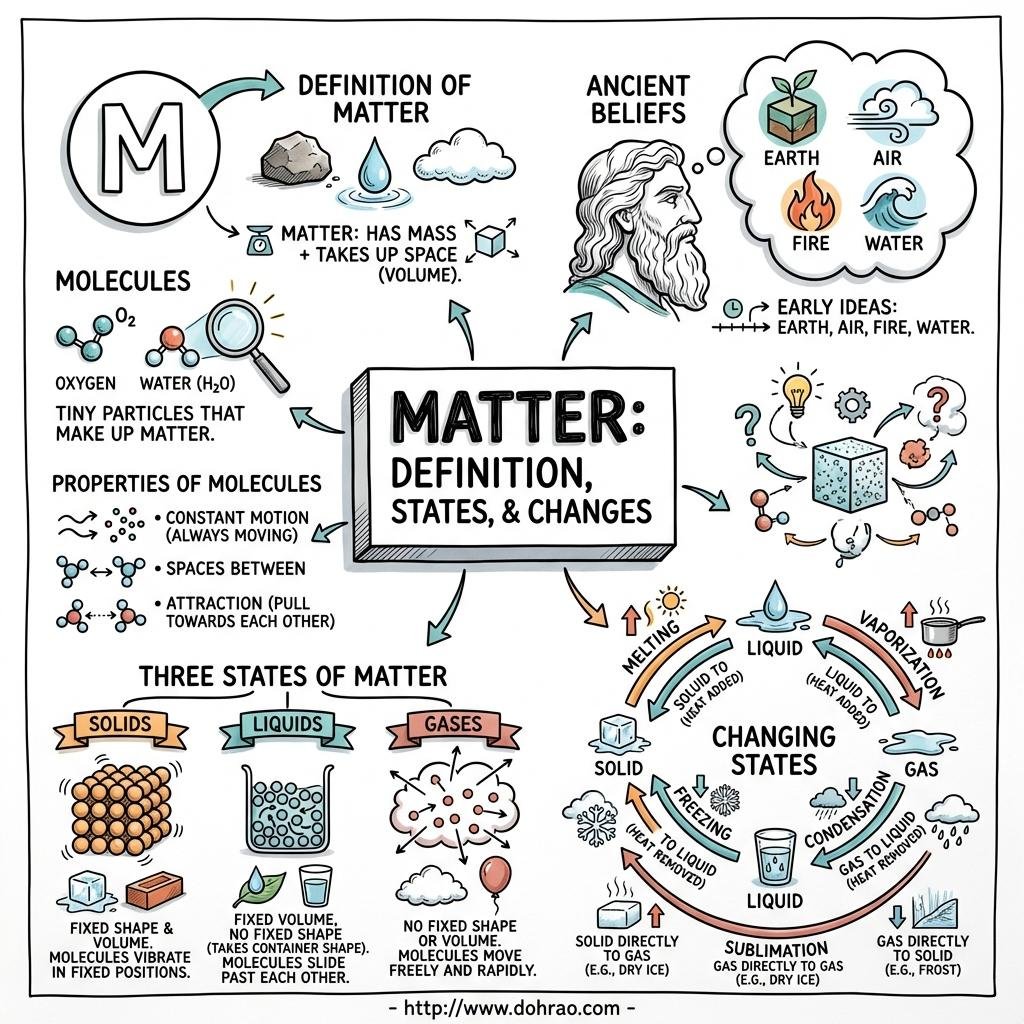
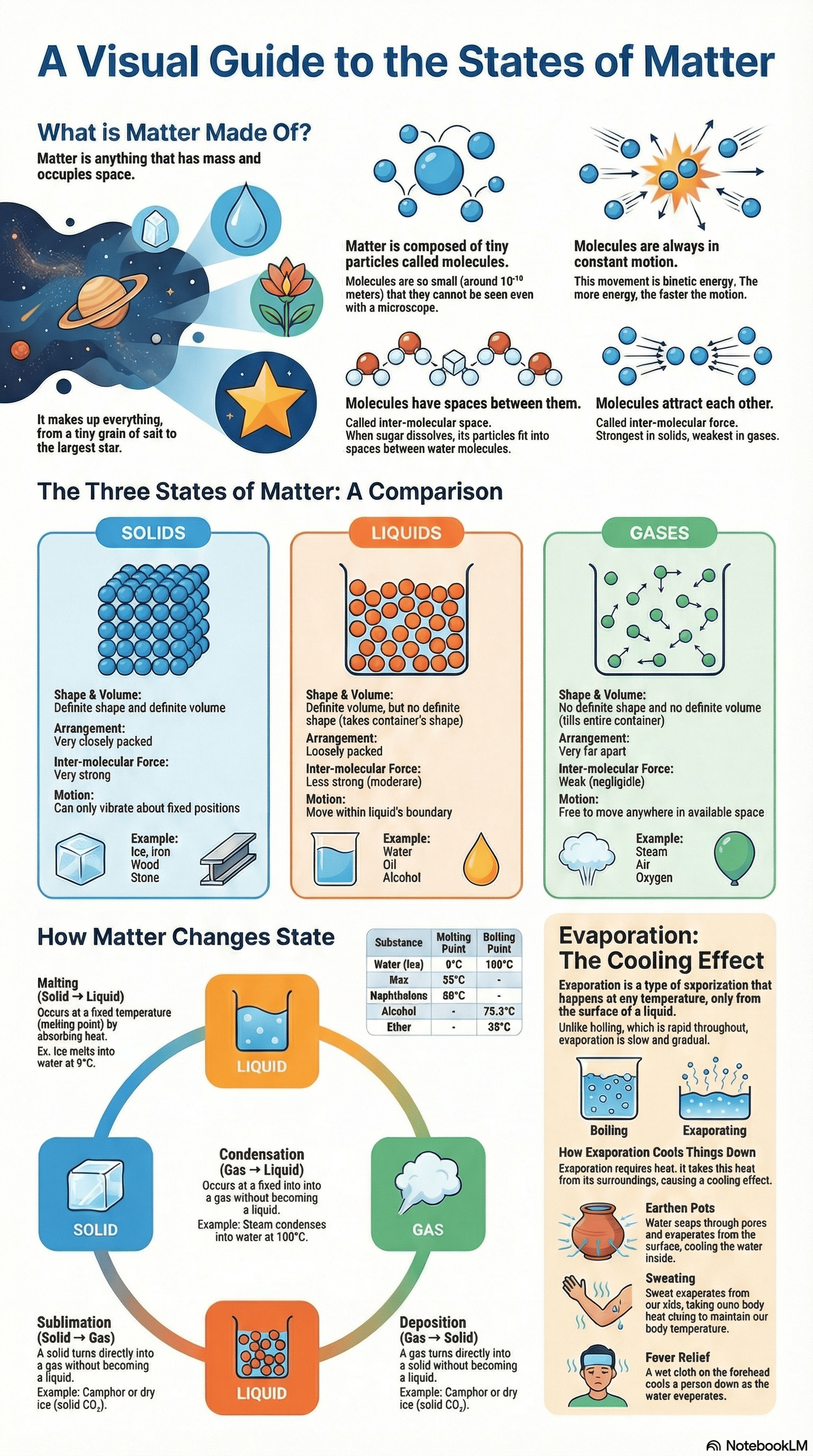
- Matter is defined as anything that occupies space, has mass, and can be perceived by our senses.
- All matter is composed of tiny particles called molecules, which are the smallest units that can exist independently and retain all properties of a substance.
- Molecules are made of atoms; they can be monoatomic (one atom), diatomic (two atoms), or polyatomic (more than two atoms).
2. Characteristics of Molecules
- Extremely Small Size: Molecules are approximately $10^{-10}$ meters in size.
- Inter-molecular Space: There are spaces between molecules, which are least in solids and maximum in gases.
- Constant Motion: Molecules are always in motion and thus possess kinetic energy. In solids, they only vibrate, while in gases, they move randomly.
- Inter-molecular Force: Molecules exert an attractive force on each other. Force of cohesion exists between similar molecules, while force of adhesion exists between different types of molecules.
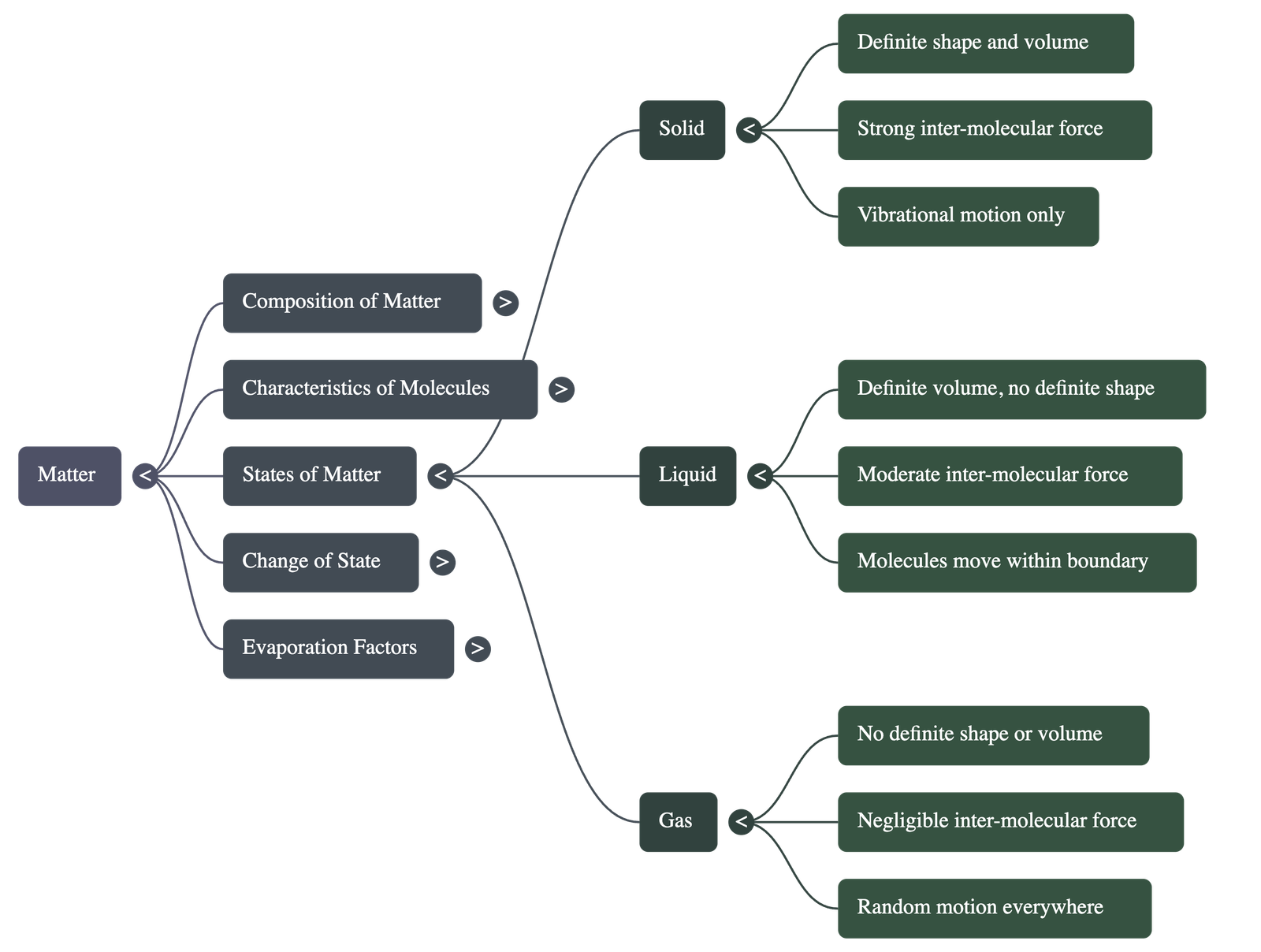
3. The Three States of Matter
- Solids: They are rigid with a definite shape and volume because their molecules are closely packed with very strong attractive forces.
- Liquids: They have a definite volume but no definite shape (they take the shape of their container) because molecules are less closely packed and can move within a boundary.
- Gases: They have neither a definite shape nor volume because the inter-molecular forces are negligible, allowing molecules to move freely anywhere.
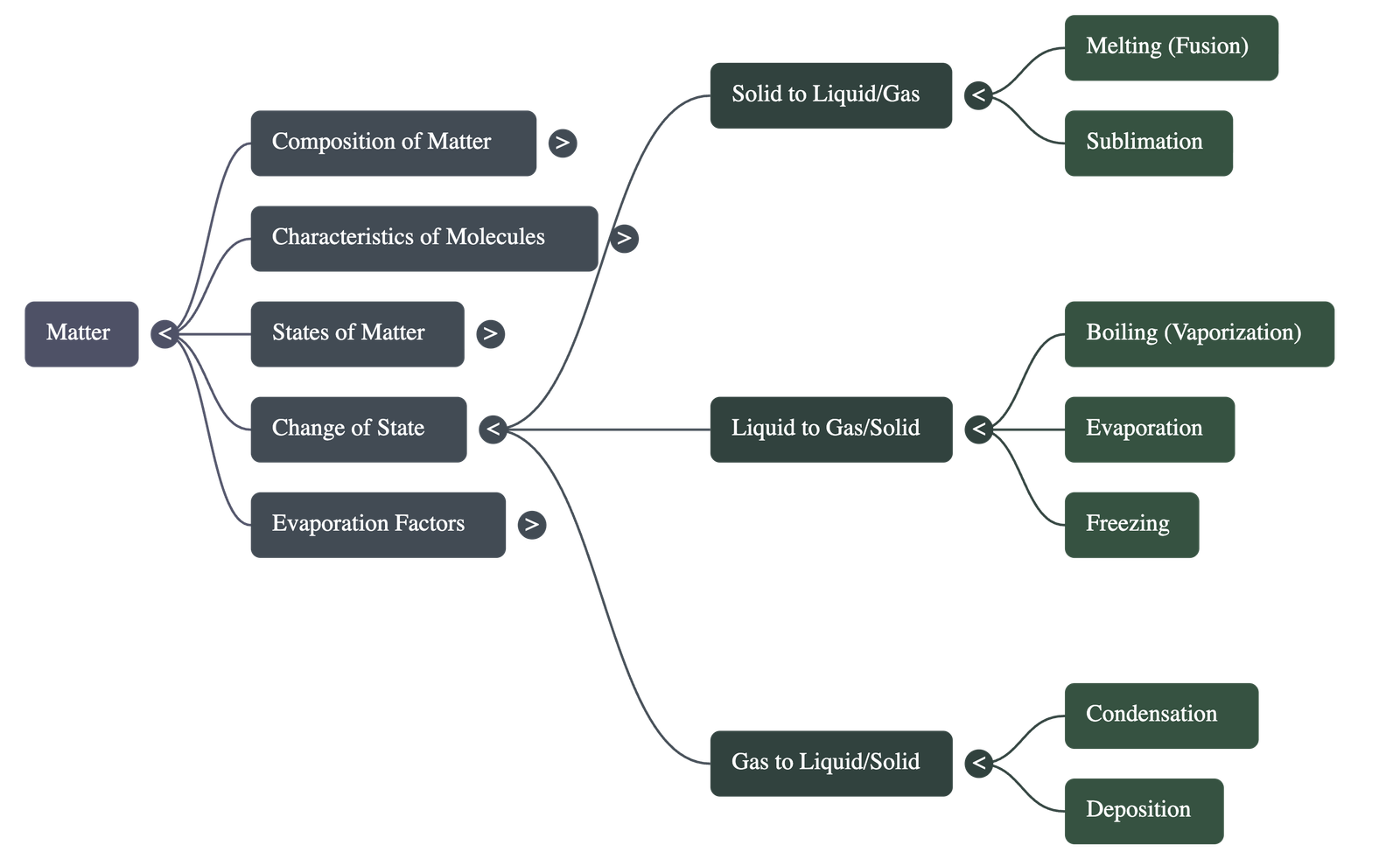
4. Change of State and Latent Heat
- A change of state is the process of moving from one physical form to another by absorbing or rejecting heat at a constant temperature.
- Latent Heat: During a state change, the heat absorbed or rejected does not change the substance's temperature; instead, it is used to change the separation (potential energy) between molecules.
- Melting and Freezing: Melting is the transition from solid to liquid at a fixed "melting point," while freezing is the reverse. For a specific substance, the melting and freezing points are the same (e.g., $0^\circ\text{C}$ for water).
- Vaporization and Condensation: Vaporization (boiling) is the change from liquid to gas at a fixed "boiling point," while condensation is the reverse.
5. Sublimation and Deposition
- Sublimation is the process where a solid changes directly into a gas without becoming a liquid (e.g., camphor, naphthalene).
- Deposition (or solidification) is the reverse process where a gas changes directly into a solid upon cooling.
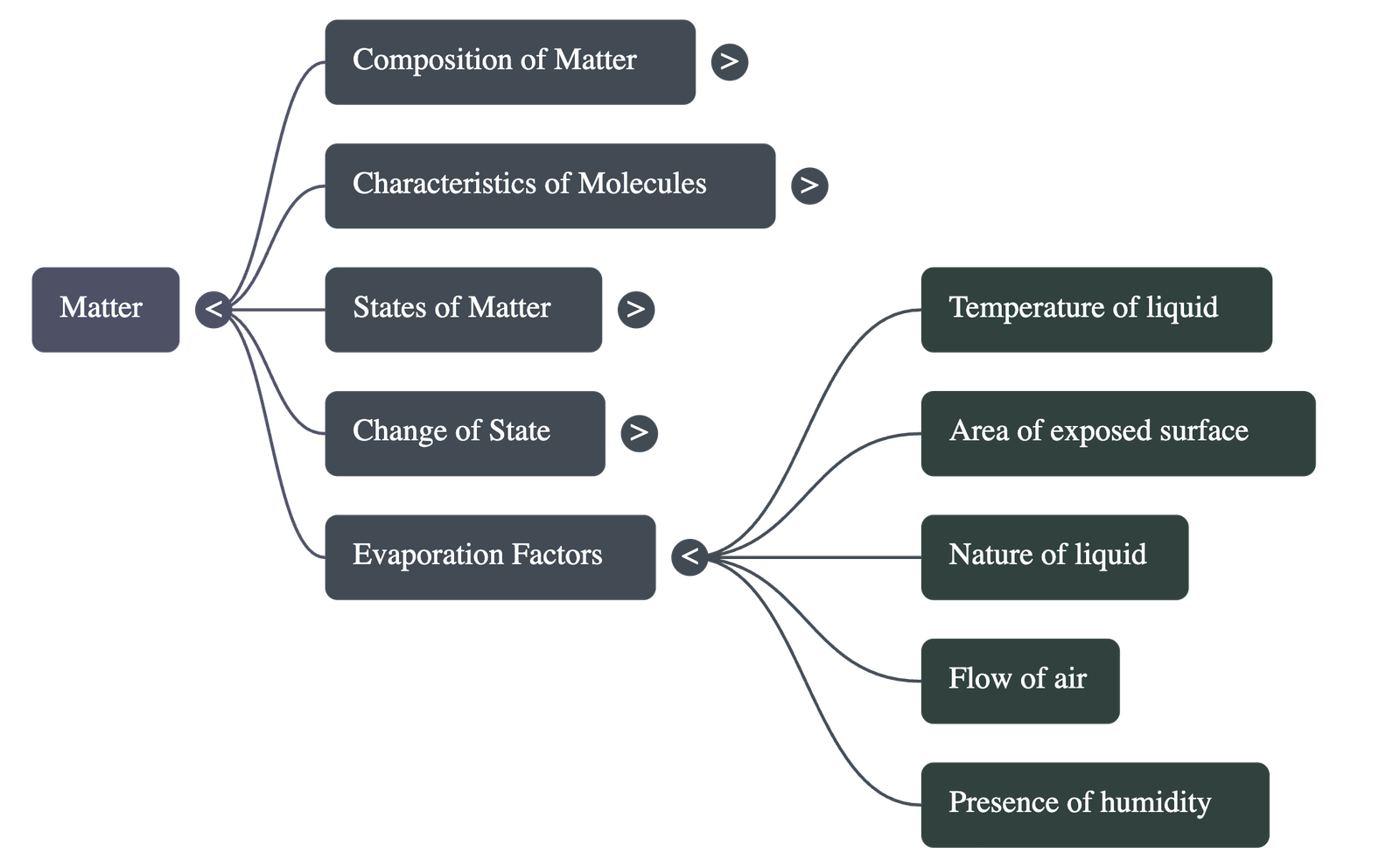
6. Evaporation
- Unlike boiling, evaporation is a slow process that occurs at all temperatures and only from the surface of a liquid.
- Factors affecting evaporation: It increases with higher temperature, larger surface area, increased air flow (wind), and lower humidity.
- Cooling Effect: Evaporation produces cooling because the liquid absorbs the necessary heat for the process from its surroundings.
To visualize these concepts, you might think of the molecules as people in different social settings: in a solid, they are like a crowd in a packed elevator (stuck in place but vibrating); in a liquid, they are like people at a busy party (moving around but staying within the room); and in a gas, they are like lone runners in a vast open park (moving freely and rarely interacting).
Quick Navigation:
| | | | |
1 / 1
Quick Navigation:
| | | | |