Quick Navigation:
| | | | |
Quick Navigation:
| | | | |
Based on the provided sources, here is a point-wise summary of Chapter 2, Physical Quantities and Measurement:
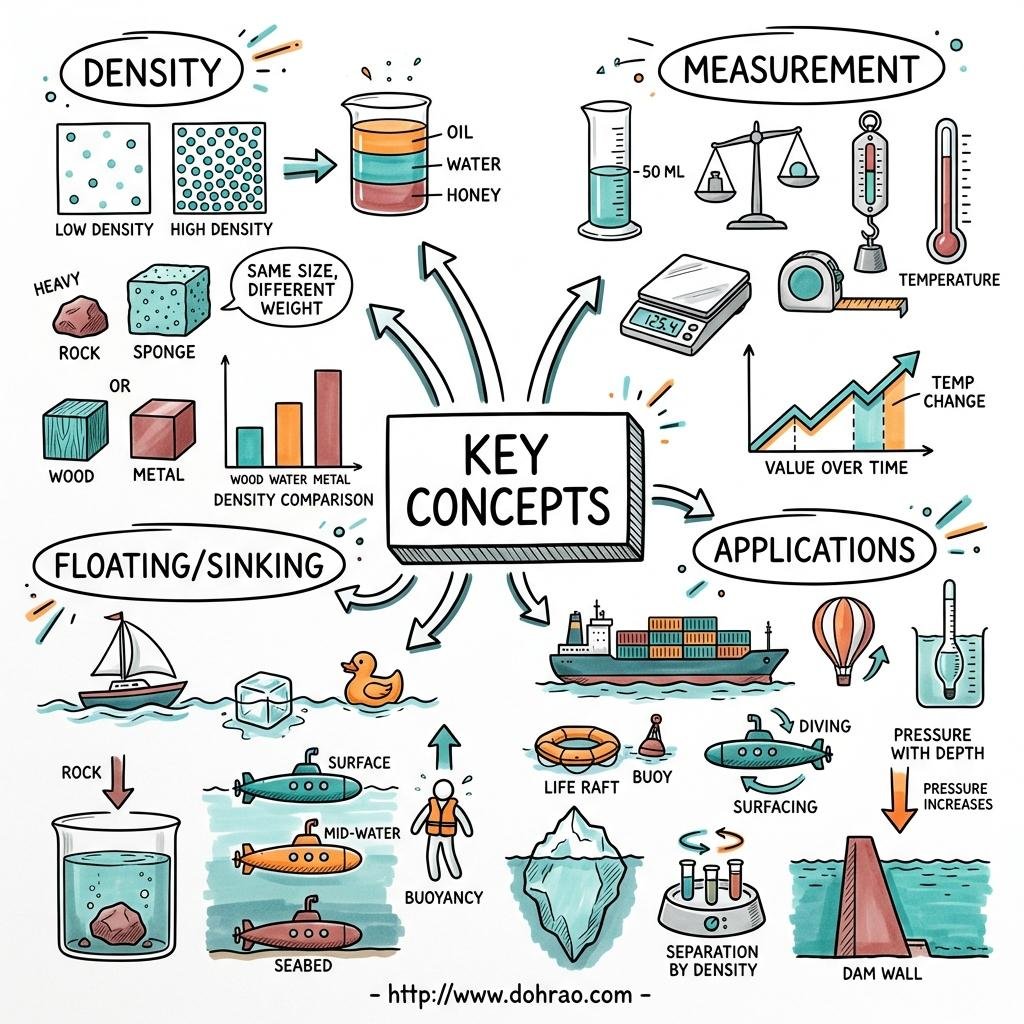
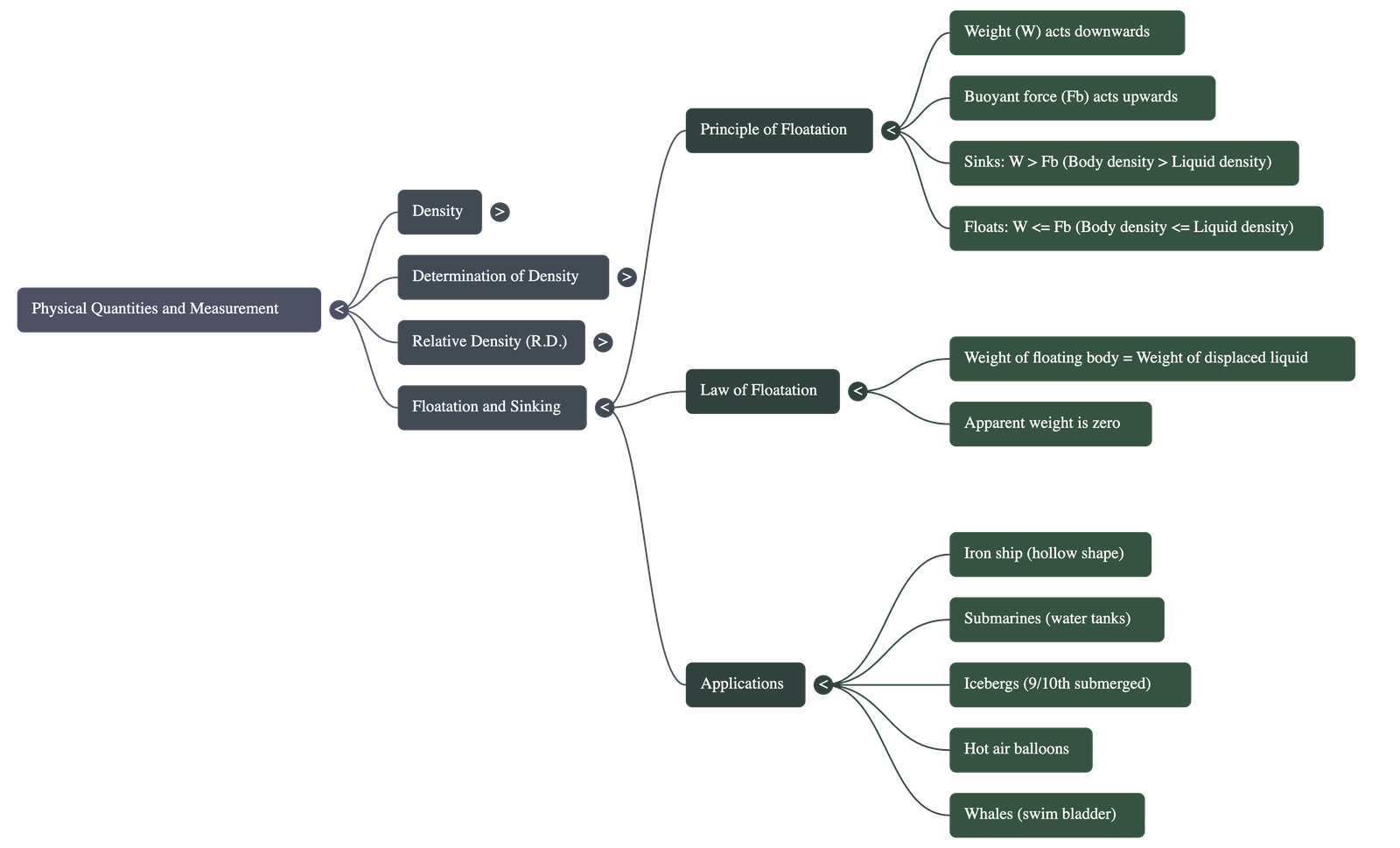
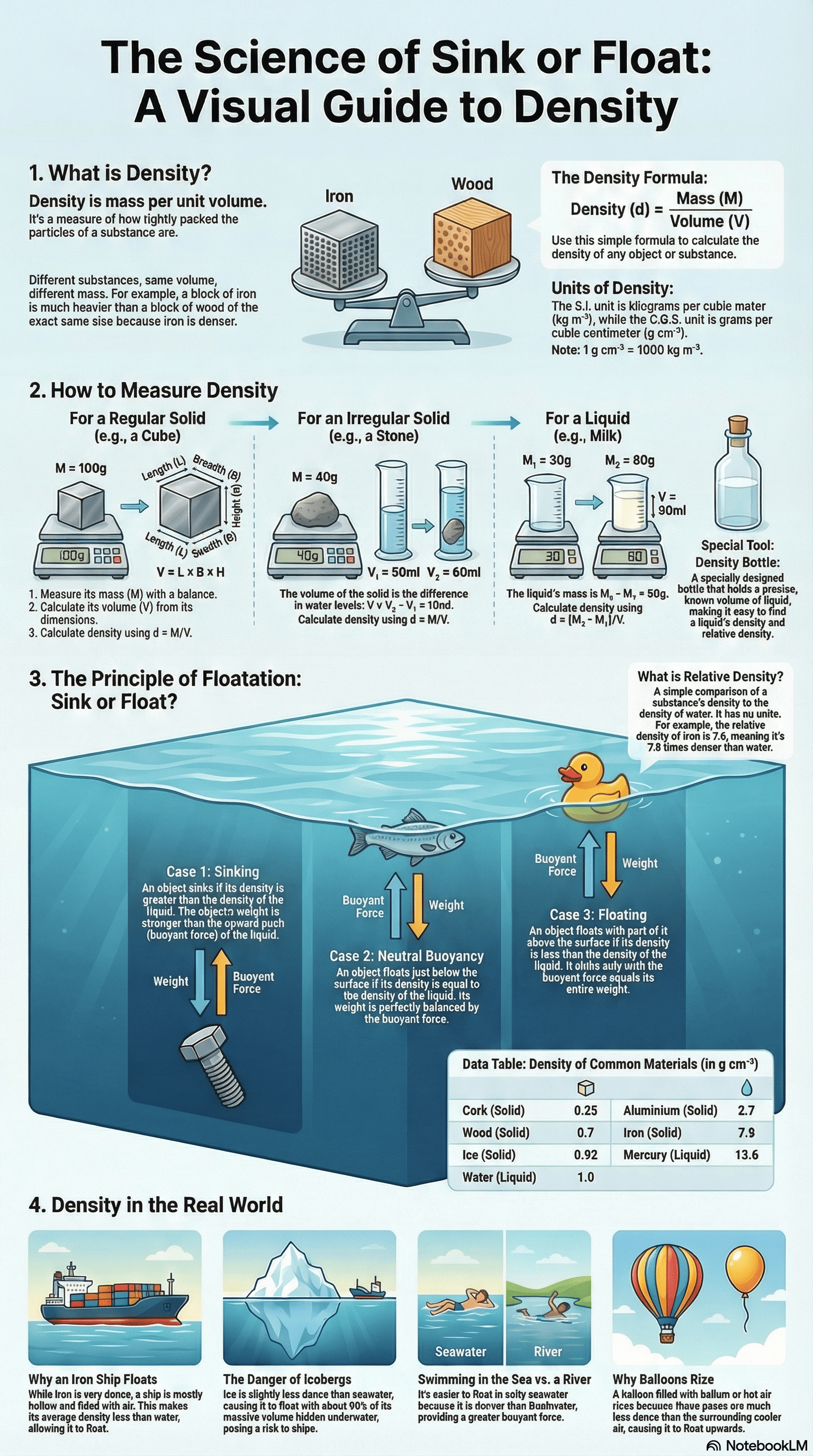
1. Concept of Density
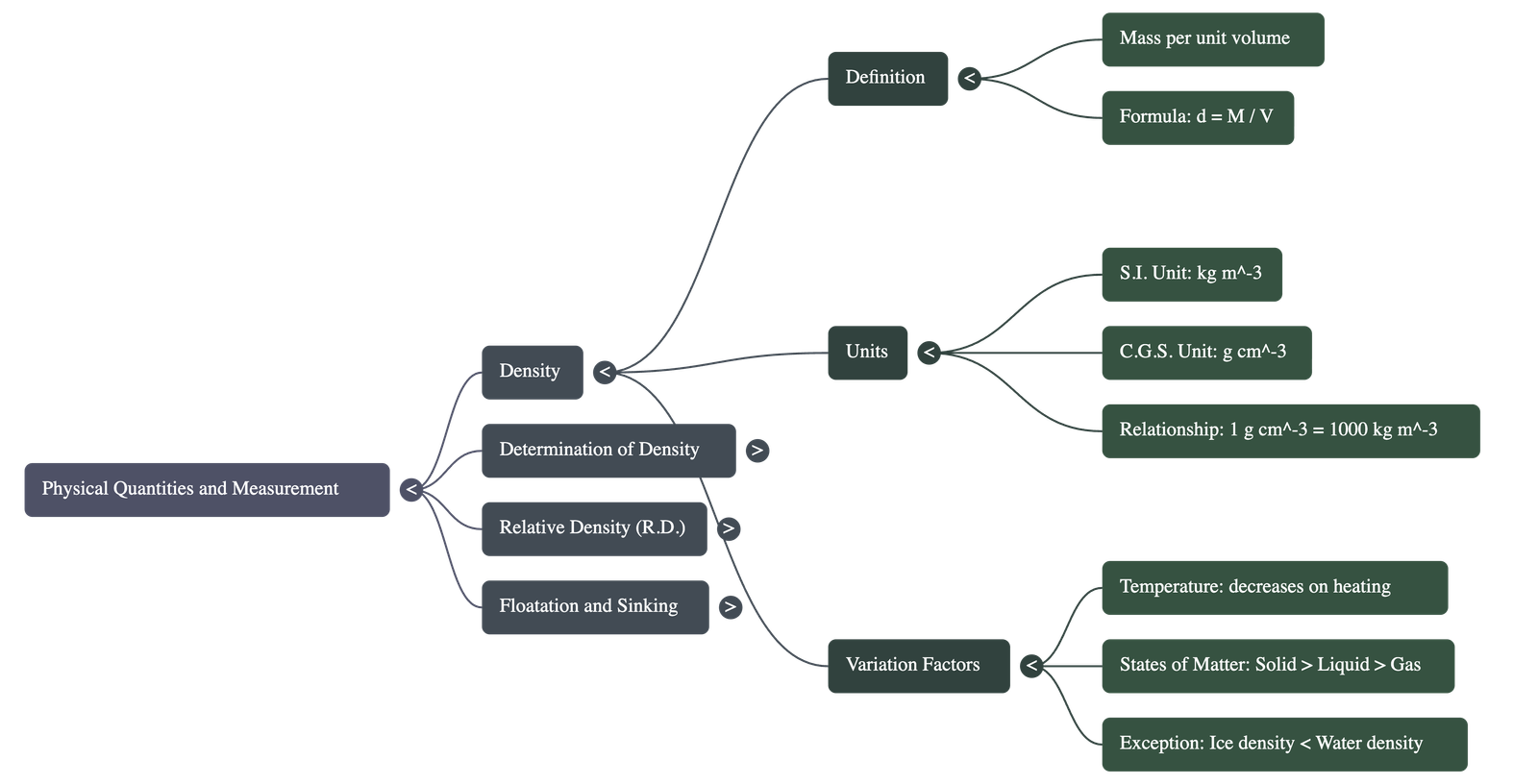
- Definition: Density is defined as the mass of a substance per unit volume ($d = M/V$).
- Variation: Different substances have different masses for the same volume, or different volumes for the same mass, due to how closely their particles are packed.
- Units: The S.I. unit is $\text{kg m}^{-3}$ (kilogram per cubic metre), and the C.G.S. unit is $\text{g cm}^{-3}$ (gram per cubic centimetre).
- Relationship: $1 \text{ g cm}^{-3} = 1000 \text{ kg m}^{-3}$.
- Effect of Temperature: Generally, density decreases as temperature increases because substances expand while mass stays constant.
- Water's Anomaly: Water is an exception; its density increases from $0^{\circ}\text{C}$ to $4^{\circ}\text{C}$ and then decreases above $4^{\circ}\text{C}$. It has maximum density at $4^{\circ}\text{C}$ ($1000 \text{ kg m}^{-3}$).
2. Measurement of Density
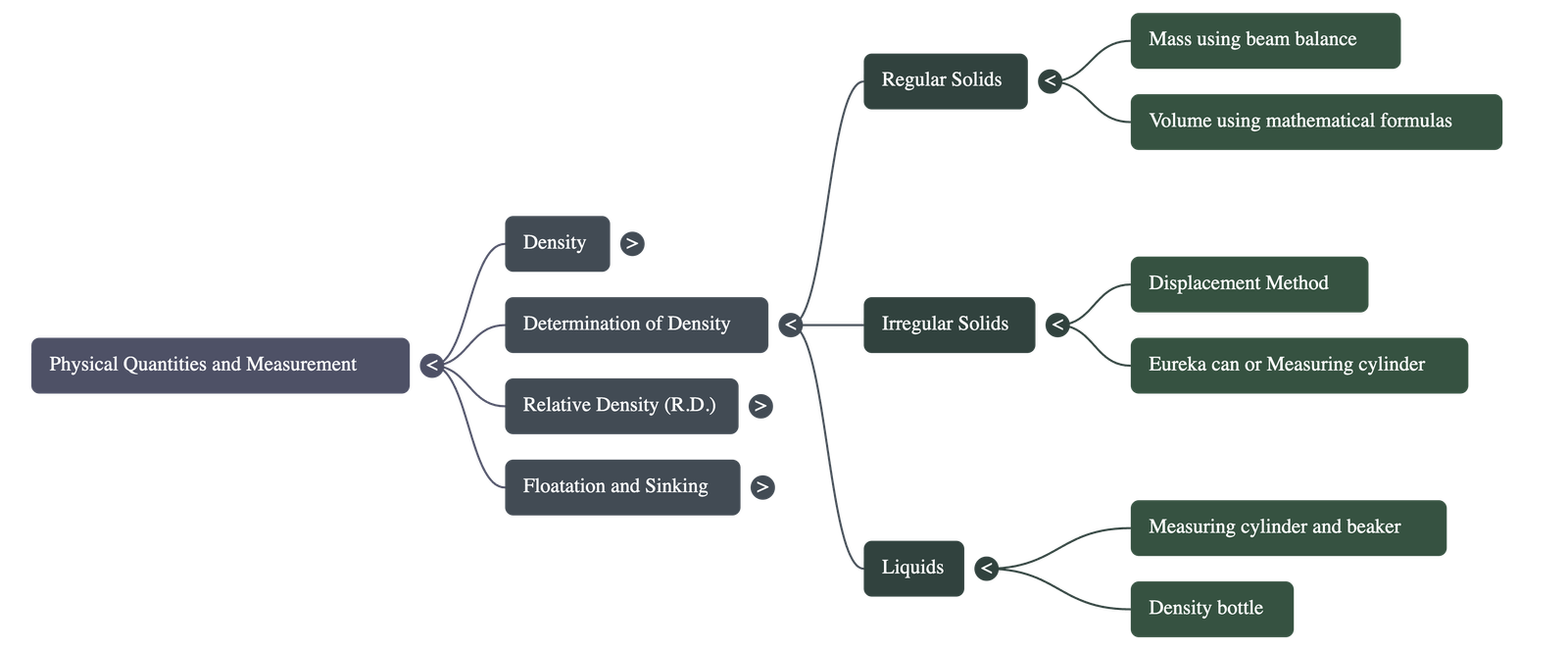
- Regular Solids: Mass is measured with a beam balance, and volume is calculated using mathematical formulas (e.g., $V = \text{side}^3$ for a cube).
- Irregular Solids: Volume is determined using the displacement method with a measuring cylinder or a Eureka can. The volume of the displaced liquid equals the volume of the solid.
- Liquids: Density can be found using a measuring cylinder to find volume and a beam balance for mass, or more accurately using a density bottle, which stores a fixed volume of liquid.
3. Relative Density (R.D.)
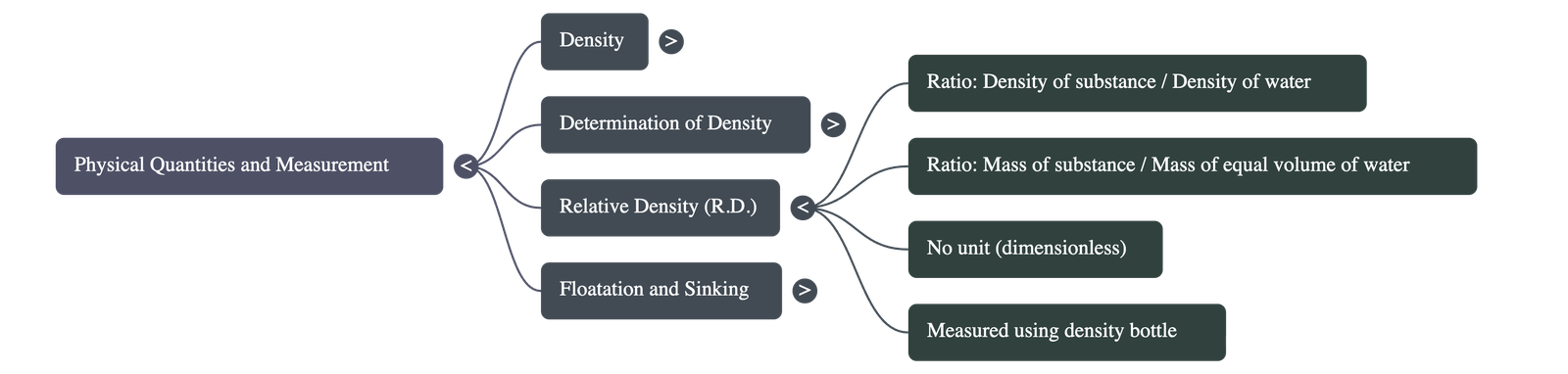
- Definition: It is the ratio of the density of a substance to the density of water at $4^{\circ}\text{C}$.
- Nature: Since it is a ratio of similar quantities, it is a dimensionless number (it has no units).
- Calculation: It can also be expressed as the ratio of the mass of any volume of a substance to the mass of an equal volume of water.
4. Density in Different States of Matter
- Generally, a substance is most dense in its solid state, less dense in its liquid state, and least dense in its gaseous state.
- Exception: Ice ($0.917 \text{ g cm}^{-3}$) is less dense than liquid water ($1.00 \text{ g cm}^{-3}$), which is why ice floats on water.
5. Floating and Sinking
- Buoyant Force (Upthrust): When a body is immersed in a liquid, it experiences an upward force equal to the weight of the liquid displaced by the body.
- Sinking: A body sinks if its density is greater than the density of the liquid (or if its weight is greater than the maximum buoyant force).
- Floating: A body floats if its density is equal to or less than the density of the liquid.
- Law of Floatation: When a body floats, the weight of the floating body is equal to the weight of the liquid displaced by its immersed part. The apparent weight of a floating body is always zero.
6. Applications of Floatation
- Iron Ships: Although iron is denser than water, a ship is hollow and contains air, making its average density less than water, allowing it to float.
- Submarines: They have ballast tanks that can be filled with water to increase average density (to sink) or emptied to decrease it (to rise).
- Icebergs: They float in sea water with about 9/10th of their volume submerged because the density of ice is slightly less than that of sea water.
- Balloons: Hydrogen or helium-filled balloons rise because the density of these gases is less than that of air, creating a net upward force.
Analogy for Buoyancy: Think of the buoyant force like a helpful friend standing in a pool. If you try to push a large beach ball under the water, the "friend" (the water) pushes back up with a lot of strength. If the object you're pushing is heavier than the "friend's" total strength, it will sink; if it's lighter, the "friend" will easily keep it at the surface.
Quick Navigation:
| | | | |
1 / 1
Quick Navigation:
| | | | |